The SH3 domain directs acto-myosin-dependent targeting of v-Src to focal adhesions via phosphatidylinositol 3-kinase - PubMed (original) (raw)
The SH3 domain directs acto-myosin-dependent targeting of v-Src to focal adhesions via phosphatidylinositol 3-kinase
V J Fincham et al. Mol Cell Biol. 2000 Sep.
Abstract
The v-Src oncoprotein is translocated to integrin-linked focal adhesions, where its tyrosine kinase activity induces adhesion disruption and cell transformation. We previously demonstrated that the intracellular targeting of Src is dependent on the actin cytoskeleton, under the control of the Rho family of small G proteins. However, the assembly of v-Src into focal adhesions does not require its catalytic activity or myristylation-dependent membrane association. Here, we report that the SH3 domain is essential for the assembly of focal adhesions containing the oncoprotein by mediating a switch from a microtubule-dependent, perinuclear localization to actin-associated focal adhesions; furthermore, v-Src translocation to focal adhesions requires myosin activity, at least under normal conditions when the actin cytoskeleton is being dynamically regulated. Although the SH3 domain of v-Src is also necessary for its association with focal adhesion kinase (FAK), which is often considered a likely candidate mediator of focal adhesion targeting via its carboxy-terminal targeting sequence, we show here that binding to FAK is not essential for the targeting of v-Src to focal adhesions. The p85 regulatory subunit of phosphatidylinositol (PI) 3-kinase also associates with v-Src in an SH3-dependent manner, but in this case inhibition of PI 3-kinase activity suppressed assembly of focal adhesions containing the oncoprotein. Thus, the Src SH3 domain, which binds PI 3-kinase and which is necessary for activation of Akt downstream, is required for the actin-dependent targeting of v-Src to focal adhesions.
Figures
FIG. 1
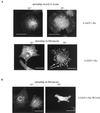
The SH3 domain of _ts LA_29 v-Src is required for translocation and actin association. (A) CE cells expressing _ts LA_29 v-Src, its KD variant _ts LA_29 v-Src-KD, or _ts LA_29 v-Src which has a point mutation in the SH3 domain (W118A) were maintained at 41°C or were shifted to 35°C for 2, 7, or 24 h prior to staining with anti-Src (and antipaxillin for cells after 24 h at 35°C) and a secondary antibody coupled to Texas red or FITC. Bars, 25 μm. (B) CE cells expressing _ts LA_29 v-Src or its kinase-defective variant, _ts LA_29 v-Src-KD, or the SH3 domain mutant, _ts LA_29 v-Src-W118A were maintained at 41°C or were shifted to 35°C for 30 or 60 min. Cytoskeletons were extracted and subjected to two rounds of partial depolymerization and repolymerization as described in Materials and Methods. After the final polymerization, preparations were solubilized and proteins were separated by SDS–10% PAGE and immunoblotted using anti-Src or antiactin as the probe. As a control (B, top left), a whole-cell lysate of ts v-Src-expressing cells grown at 35°C was immunoblotted with anti-Src. (C) Whole-cell lysates of _ts LA_29 v-Src, _ts LA_29 v-Src-KD-, or _ts LA_29 v-Src-W118A-expressing cells were immunoblotted using anti-Src-phospho-Tyr-416 (upper) or anti-Src (lower) as the probe. The positions of v-Src are indicated. CEF, CE fibroblasts.
FIG. 1
The SH3 domain of _ts LA_29 v-Src is required for translocation and actin association. (A) CE cells expressing _ts LA_29 v-Src, its KD variant _ts LA_29 v-Src-KD, or _ts LA_29 v-Src which has a point mutation in the SH3 domain (W118A) were maintained at 41°C or were shifted to 35°C for 2, 7, or 24 h prior to staining with anti-Src (and antipaxillin for cells after 24 h at 35°C) and a secondary antibody coupled to Texas red or FITC. Bars, 25 μm. (B) CE cells expressing _ts LA_29 v-Src or its kinase-defective variant, _ts LA_29 v-Src-KD, or the SH3 domain mutant, _ts LA_29 v-Src-W118A were maintained at 41°C or were shifted to 35°C for 30 or 60 min. Cytoskeletons were extracted and subjected to two rounds of partial depolymerization and repolymerization as described in Materials and Methods. After the final polymerization, preparations were solubilized and proteins were separated by SDS–10% PAGE and immunoblotted using anti-Src or antiactin as the probe. As a control (B, top left), a whole-cell lysate of ts v-Src-expressing cells grown at 35°C was immunoblotted with anti-Src. (C) Whole-cell lysates of _ts LA_29 v-Src, _ts LA_29 v-Src-KD-, or _ts LA_29 v-Src-W118A-expressing cells were immunoblotted using anti-Src-phospho-Tyr-416 (upper) or anti-Src (lower) as the probe. The positions of v-Src are indicated. CEF, CE fibroblasts.
FIG. 2
The peripheral targeting of v-Src is integrin dependent. (A) CE cells expressing _ts LA_29 v-Src that had been grown at 41°C were trypsinized, held in suspension, and plated onto poly-
l
-lysine- or fibronectin-coated dishes for 40 min at either 41 or 35°C. Fixed cells were stained with anti-Src (and a secondary antibody coupled to FITC). (B) CE cells expressing the SH3 domain mutant, _ts LA_29 v-Src-W118A, were plated onto fibronectin for 40 min at either 41 or 35°C. (C) CE cells expressing _ts LA_29 v-Src or the SH3 domain mutant, _ts LA_29 v-Src-W118A, were plated onto fibronectin for 40 min at 35°C. Fixed cells were stained with anti-Src and antipaxillin (and secondary antibodies coupled to Texas red or FITC). Broken arrows indicate small structures at all periphery; some paxillin staining is visible in the lower right panel. Bars, 25 μm.
FIG. 2
The peripheral targeting of v-Src is integrin dependent. (A) CE cells expressing _ts LA_29 v-Src that had been grown at 41°C were trypsinized, held in suspension, and plated onto poly-
l
-lysine- or fibronectin-coated dishes for 40 min at either 41 or 35°C. Fixed cells were stained with anti-Src (and a secondary antibody coupled to FITC). (B) CE cells expressing the SH3 domain mutant, _ts LA_29 v-Src-W118A, were plated onto fibronectin for 40 min at either 41 or 35°C. (C) CE cells expressing _ts LA_29 v-Src or the SH3 domain mutant, _ts LA_29 v-Src-W118A, were plated onto fibronectin for 40 min at 35°C. Fixed cells were stained with anti-Src and antipaxillin (and secondary antibodies coupled to Texas red or FITC). Broken arrows indicate small structures at all periphery; some paxillin staining is visible in the lower right panel. Bars, 25 μm.
FIG. 3
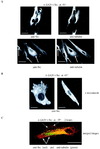
Inactive v-Src colocalizes with regions of dense tubulin staining and switches to actin after activation. Confocal images are of CE cells expressing _ts LA_29 v-Src stained with anti-Src (and a secondary antibody coupled to Texas red) and anti-α-tubulin (and a secondary antibody coupled to FITC). v-Src was maintained in the inactive state at 41°C, and cells were either untreated (A) or treated with 0.5 μg of nocodazole/ml (B). Arrows (A), regions of colocalization of v-Src and tubulin. (C) Cells were stained with anti-Src (visualized as red) and anti-α-tubulin (visualized as green) after the switch to the permissive temperature (35°C) for 2 h. In the merged image, v-Src-staining focal adhesions at the cell periphery are indicated by arrows. (D) v-Src-containing complexes at the ends of MLC-staining filaments are shown in CE cells expressing _ts LA_29 v-Src that were shifted to 35°C for 1 h and double stained with both anti-Src (followed by a secondary antibody coupled to FITC) and anti-MLC (followed by a secondary antibody coupled to Texas red). (E) CE cells expressing _ts LA_29 v-Src were shifted to 35°C for 1 h and double stained with both anti-Src (followed by a secondary antibody coupled to FITC) and phalloidin-tetramethyl rhodamine isocyanate. Arrows, v-Src-containing complexes colocalizing with the ends of actin filaments. Bars, 25 μm.
FIG. 3
Inactive v-Src colocalizes with regions of dense tubulin staining and switches to actin after activation. Confocal images are of CE cells expressing _ts LA_29 v-Src stained with anti-Src (and a secondary antibody coupled to Texas red) and anti-α-tubulin (and a secondary antibody coupled to FITC). v-Src was maintained in the inactive state at 41°C, and cells were either untreated (A) or treated with 0.5 μg of nocodazole/ml (B). Arrows (A), regions of colocalization of v-Src and tubulin. (C) Cells were stained with anti-Src (visualized as red) and anti-α-tubulin (visualized as green) after the switch to the permissive temperature (35°C) for 2 h. In the merged image, v-Src-staining focal adhesions at the cell periphery are indicated by arrows. (D) v-Src-containing complexes at the ends of MLC-staining filaments are shown in CE cells expressing _ts LA_29 v-Src that were shifted to 35°C for 1 h and double stained with both anti-Src (followed by a secondary antibody coupled to FITC) and anti-MLC (followed by a secondary antibody coupled to Texas red). (E) CE cells expressing _ts LA_29 v-Src were shifted to 35°C for 1 h and double stained with both anti-Src (followed by a secondary antibody coupled to FITC) and phalloidin-tetramethyl rhodamine isocyanate. Arrows, v-Src-containing complexes colocalizing with the ends of actin filaments. Bars, 25 μm.
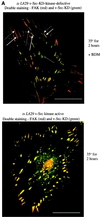
FIG. 4
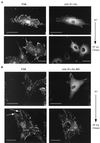
The myosin ATPase inhibitor BDM and the MLCK inhibitor ML7 block kinase-active v-Src translocation. CE cells expressing _ts LA_29 v-Src (A) or KD _ts LA_29 v-Src-KD (B) were maintained at 41°C or shifted to 35°C for 2 h in the presence of BDM and stained with anti-Src (followed by a secondary antibody coupled to FITC; right) and anti-FAK (followed by a secondary antibody coupled to Texas red; left). Arrows, enlarged peripheral adhesions. (C) Cells expressing _ts LA_29 v-Src (upper) or _ts LA_29 v-Src-KD (lower) after the shift to 35°C for 2 h in the absence (−) or presence (+) of ML7 were stained with anti-Src (followed by a secondary antibody coupled to FITC). Bars, 25 μm.
FIG. 4
The myosin ATPase inhibitor BDM and the MLCK inhibitor ML7 block kinase-active v-Src translocation. CE cells expressing _ts LA_29 v-Src (A) or KD _ts LA_29 v-Src-KD (B) were maintained at 41°C or shifted to 35°C for 2 h in the presence of BDM and stained with anti-Src (followed by a secondary antibody coupled to FITC; right) and anti-FAK (followed by a secondary antibody coupled to Texas red; left). Arrows, enlarged peripheral adhesions. (C) Cells expressing _ts LA_29 v-Src (upper) or _ts LA_29 v-Src-KD (lower) after the shift to 35°C for 2 h in the absence (−) or presence (+) of ML7 were stained with anti-Src (followed by a secondary antibody coupled to FITC). Bars, 25 μm.
FIG. 5
Actin stress fiber stability is unaffected by BDM in cells expressing KD _ts LA_29 v-Src at the permissive temperature. CE cells expressing either _ts LA_29 v-Src or KD _ts LA_29 v-Src-KD were maintained at 41°C or shifted to 35°C for 2 h and stained with phalloidin-FITC to visualize actin stress fibers. Cells were either untreated (− BDM; A) or treated with 10 mM BDM for 2 h at 41°C or during the 2-h period of the shift to 35°C (+ BDM; B).
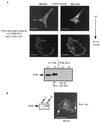
FIG. 6
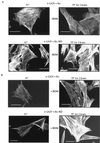
KD v-Src can assemble into preexisting focal adhesions. (A) Merged confocal images shown in Fig. 4B representing CE cells expressing KD _ts LA_29 v-Src-KD (upper) that were shifted to 35°C for 2 h in the presence of BDM (+ BDM) and stained with both anti-FAK (and a secondary antibody coupled to Texas red; visualized as red) and anti-Src (and a secondary antibody coupled to FITC; visualized as green). Grey arrow, intracellular complexes containing both FAK and v-Src (visualized as yellow); broken white arrows, enlarged peripheral adhesions containing FAK at the membrane-proximal region (visualized as red); solid white arrows, enlarged peripheral adhesions containing both FAK and v-Src at the membrane-distal region (visualized as yellow). Also shown are merged confocal images after dual staining of kinase-active _ts LA_29 v-Src-expressing cells (lower) that were shifted for 2 h to 35°C with anti-FAK and anti-Src. Colocalization of v-Src and FAK is visualized as yellow. (B) Merged confocal images after dual staining of KD _ts LA_29 v-Src-KD-expressing cells (upper) or kinase-active _ts LA_29 v-Src (lower) that were shifted for 2 h to 35°C with antipaxillin (and a secondary antibody coupled to Texas red) and anti-Src (and a secondary antibody coupled to FITC). Focal adhesions that stain with paxillin (visualized as red) in the membrane-proximal region (broken arrows) and both Src and paxillin (visualized as yellow) in the membrane-distal region (solid arrows) are shown. Bars, 25 μm.
FIG. 6
KD v-Src can assemble into preexisting focal adhesions. (A) Merged confocal images shown in Fig. 4B representing CE cells expressing KD _ts LA_29 v-Src-KD (upper) that were shifted to 35°C for 2 h in the presence of BDM (+ BDM) and stained with both anti-FAK (and a secondary antibody coupled to Texas red; visualized as red) and anti-Src (and a secondary antibody coupled to FITC; visualized as green). Grey arrow, intracellular complexes containing both FAK and v-Src (visualized as yellow); broken white arrows, enlarged peripheral adhesions containing FAK at the membrane-proximal region (visualized as red); solid white arrows, enlarged peripheral adhesions containing both FAK and v-Src at the membrane-distal region (visualized as yellow). Also shown are merged confocal images after dual staining of kinase-active _ts LA_29 v-Src-expressing cells (lower) that were shifted for 2 h to 35°C with anti-FAK and anti-Src. Colocalization of v-Src and FAK is visualized as yellow. (B) Merged confocal images after dual staining of KD _ts LA_29 v-Src-KD-expressing cells (upper) or kinase-active _ts LA_29 v-Src (lower) that were shifted for 2 h to 35°C with antipaxillin (and a secondary antibody coupled to Texas red) and anti-Src (and a secondary antibody coupled to FITC). Focal adhesions that stain with paxillin (visualized as red) in the membrane-proximal region (broken arrows) and both Src and paxillin (visualized as yellow) in the membrane-distal region (solid arrows) are shown. Bars, 25 μm.
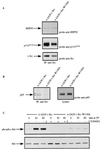
FIG. 7
FAK binding v-Src via the Src SH3 domain is not required for translocation. (A) v-Src was immunoprecipitated (IP) from lysates of cells expressing _ts LA_29 v-Src or _ts LA_29 v-Src-W118A (-W118A) at 41°C and after a shift to 35°C for 2 h. Precipitated proteins were immunoblotted using both anti-FAK and anti-Src as probes. The position of FAK coprecipitating with v-Src is indicated. Immunoprecipitated v-Src is also indicated. The positions of the 97- and 69-kDa molecular mass markers are shown. NI, nonimmune serum. (B) Lysates of CE cells expressing _ts LA_29 v-Src either alone (−) or together with wild-type _myc_-tagged FAK (wt) or a mutant in which Tyr-397 was replaced by Phe (397F) were immunoblotted using anti-Src, anti-myc, or anti-FAK as the probe. The exogenously expressed v-Src and FAK proteins are indicated. Endogenous FAK is not visible on the exposure shown for the anti-FAK blot, demonstrating the overexpression of exogenous retrovirus-encoded FAK proteins (endogenous FAK is visible on longer exposures [not shown]). (C) CE cells expressing both _ts LA_29 v-Src and _myc_-tagged wild-type FAK or 397F-FAK were maintained at 41°C or were shifted to 35°C for 2 h. Lysates were immunoprecipitated with anti-Src or anti-FAK and blotted using anti-myc as the probe. The position of _myc_-tagged FAK precipitated by anti-Src or anti-FAK is indicated. Anti-Src immunoprecipitates were also probed with anti-FAK and anti-Src. (D) CE cells expressing both _ts LA_29 v-Src and _myc_-tagged FAK-397F were maintained at 41°C or were shifted to 35°C for 2 h and were stained with anti-Src (followed by a secondary antibody coupled to Texas red) and anti-myc (followed by a secondary antibody coupled to FITC). Bars, 25 μm.
FIG. 7
FAK binding v-Src via the Src SH3 domain is not required for translocation. (A) v-Src was immunoprecipitated (IP) from lysates of cells expressing _ts LA_29 v-Src or _ts LA_29 v-Src-W118A (-W118A) at 41°C and after a shift to 35°C for 2 h. Precipitated proteins were immunoblotted using both anti-FAK and anti-Src as probes. The position of FAK coprecipitating with v-Src is indicated. Immunoprecipitated v-Src is also indicated. The positions of the 97- and 69-kDa molecular mass markers are shown. NI, nonimmune serum. (B) Lysates of CE cells expressing _ts LA_29 v-Src either alone (−) or together with wild-type _myc_-tagged FAK (wt) or a mutant in which Tyr-397 was replaced by Phe (397F) were immunoblotted using anti-Src, anti-myc, or anti-FAK as the probe. The exogenously expressed v-Src and FAK proteins are indicated. Endogenous FAK is not visible on the exposure shown for the anti-FAK blot, demonstrating the overexpression of exogenous retrovirus-encoded FAK proteins (endogenous FAK is visible on longer exposures [not shown]). (C) CE cells expressing both _ts LA_29 v-Src and _myc_-tagged wild-type FAK or 397F-FAK were maintained at 41°C or were shifted to 35°C for 2 h. Lysates were immunoprecipitated with anti-Src or anti-FAK and blotted using anti-myc as the probe. The position of _myc_-tagged FAK precipitated by anti-Src or anti-FAK is indicated. Anti-Src immunoprecipitates were also probed with anti-FAK and anti-Src. (D) CE cells expressing both _ts LA_29 v-Src and _myc_-tagged FAK-397F were maintained at 41°C or were shifted to 35°C for 2 h and were stained with anti-Src (followed by a secondary antibody coupled to Texas red) and anti-myc (followed by a secondary antibody coupled to FITC). Bars, 25 μm.
FIG. 8
FAK is not essential for v-Src translocation. CE cells expressing both _ts LA_29 v-Src and the FAK-Pro2 mutant were maintained at 41°C or were shifted to 35°C for 2 h and stained with anti-Src (followed by a secondary antibody coupled to Texas red) and anti-myc (followed by a secondary antibody coupled to FITC). To determine whether FAK was binding Src, lysates of cells expressing wild-type (wt) FAK or FAK-Pro2 were immunoprecipitated (IP) with anti-Src, blotted, and probed with anti-Myc (lower). The position of FAK is indicated. (B) FAK−/− cells were transiently transfected with the wild-type PrA strain of v-Src. Cells were fixed and stained with anti-Src (followed by a secondary antibody coupled to FITC). Arrows, focal adhesions containing v-Src. Also shown is an immunoblot using anti-FAK as the probe confirming FAK deficiency in the FAK−/− cells we used. Bars, 25 μm.
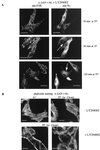
FIG. 9
The SH3 mutant v-Src protein binds HSP90 and p110AFAP but not the p85 regulatory subunit of PI 3-kinase. (A) Lysates of CE cells expressing _ts LA_29 v-Src or the SH3 mutant, _ts LA_29 v-Src-W118A, at 35°C were immunoprecipitated (IP) with anti-Src and immunoblotted using anti-HSP90, anti-p110AFAP, or anti-Src as the probe. The positions of the individual immunoprecipitated proteins are shown. (B) Lysates of CE cells expressing _ts LA_29 v-Src or the SH3 mutant, _ts LA_29 v-Src-W118A, at 35°C were immunoprecipitated with anti-Src and immunoblotted using anti-p85 as the probe (left). For comparison, the expression of both anti-p85 reactive forms was examined by immunoblotting whole-cell lysates using anti-p85 as the probe (right). (C) Lysates of cells expressing _ts LA_29 v-Src or the SH3 mutant, _ts LA_29 v-Src-W118A, that were maintained at 41°C or shifted to 35°C for 30 or 60 min (in the absence [−] or presence [+] of LY294002) were immunoblotted using anti-Akt-phospho-Ser-473 (upper) or anti-Akt (lower) as the probe. The positions of phosphorylated Akt (phospho-Akt) and Akt are indicated.
FIG. 10
The selective PI 3-kinase inhibitor LY294002 suppresses targeting of v-Src to focal adhesions. (A) Cells expressing _ts LA_29 v-Src at 30, 60, or 120 min after the switch to 35°C in the presence of LY294002 were fixed and stained with anti-FAK (followed by a secondary antibody coupled to Texas red) and anti-Src (followed by a secondary antibody coupled to FITC). The retention of v-Src in the cell interior is indicated (arrows). (B) Cells expressing _ts LA_29 v-Src either at 41°C or after 2 h at 35°C in the absence (−) or presence (+) of LY294002 were fixed and stained with phalloidin-FITC. Bars, 25 μm.
Similar articles
- v-Crk activates the phosphoinositide 3-kinase/AKT pathway by utilizing focal adhesion kinase and H-Ras.
Akagi T, Murata K, Shishido T, Hanafusa H. Akagi T, et al. Mol Cell Biol. 2002 Oct;22(20):7015-23. doi: 10.1128/MCB.22.20.7015-7023.2002. Mol Cell Biol. 2002. PMID: 12242282 Free PMC article. - Activation of Rho-dependent cell spreading and focal adhesion biogenesis by the v-Crk adaptor protein.
Altun-Gultekin ZF, Chandriani S, Bougeret C, Ishizaki T, Narumiya S, de Graaf P, Van Bergen en Henegouwen P, Hanafusa H, Wagner JA, Birge RB. Altun-Gultekin ZF, et al. Mol Cell Biol. 1998 May;18(5):3044-58. doi: 10.1128/MCB.18.5.3044. Mol Cell Biol. 1998. PMID: 9566923 Free PMC article. - v-Src's hold over actin and cell adhesions.
Frame MC, Fincham VJ, Carragher NO, Wyke JA. Frame MC, et al. Nat Rev Mol Cell Biol. 2002 Apr;3(4):233-45. doi: 10.1038/nrm779. Nat Rev Mol Cell Biol. 2002. PMID: 11994743 Review. - Actin cytoskeleton organization in response to integrin-mediated adhesion.
Defilippi P, Olivo C, Venturino M, Dolce L, Silengo L, Tarone G. Defilippi P, et al. Microsc Res Tech. 1999 Oct 1;47(1):67-78. doi: 10.1002/(SICI)1097-0029(19991001)47:1<67::AID-JEMT7>3.0.CO;2-P. Microsc Res Tech. 1999. PMID: 10506763 Review.
Cited by
- Palladin interacts with SH3 domains of SPIN90 and Src and is required for Src-induced cytoskeletal remodeling.
Rönty M, Taivainen A, Heiska L, Otey C, Ehler E, Song WK, Carpen O. Rönty M, et al. Exp Cell Res. 2007 Jul 15;313(12):2575-85. doi: 10.1016/j.yexcr.2007.04.030. Epub 2007 May 8. Exp Cell Res. 2007. PMID: 17537434 Free PMC article. - The Rho-mDia1 pathway regulates cell polarity and focal adhesion turnover in migrating cells through mobilizing Apc and c-Src.
Yamana N, Arakawa Y, Nishino T, Kurokawa K, Tanji M, Itoh RE, Monypenny J, Ishizaki T, Bito H, Nozaki K, Hashimoto N, Matsuda M, Narumiya S. Yamana N, et al. Mol Cell Biol. 2006 Sep;26(18):6844-58. doi: 10.1128/MCB.00283-06. Mol Cell Biol. 2006. PMID: 16943426 Free PMC article. - Endosomal signaling and cell migration.
Schiefermeier N, Teis D, Huber LA. Schiefermeier N, et al. Curr Opin Cell Biol. 2011 Oct;23(5):615-20. doi: 10.1016/j.ceb.2011.04.001. Epub 2011 May 3. Curr Opin Cell Biol. 2011. PMID: 21546233 Free PMC article. Review. - Endocytosis and the Src family of non-receptor tyrosine kinases.
Reinecke J, Caplan S. Reinecke J, et al. Biomol Concepts. 2014 May;5(2):143-55. doi: 10.1515/bmc-2014-0003. Biomol Concepts. 2014. PMID: 25372749 Free PMC article. Review. - Tropomyosin Tm5NM1 spatially restricts src kinase activity through perturbation of Rab11 vesicle trafficking.
Bach CT, Murray RZ, Owen D, Gaus K, O'Neill GM. Bach CT, et al. Mol Cell Biol. 2014 Dec;34(24):4436-46. doi: 10.1128/MCB.00796-14. Epub 2014 Oct 6. Mol Cell Biol. 2014. PMID: 25288639 Free PMC article.
References
- Aplin A E, Howe A, Alahari S K, Juliano R L. Signal transduction and signal modulation by cell adhesion receptors: the role of integrins, cadherins, immunoglobulin-cell adhesion molecules, and selectins. Pharmacol Rev. 1998;50:197–263. - PubMed
- Beug H, Claviez M, Jockusch B M, Graf T. Differential expression of Rous sarcoma virus-specific transformation parameters in enucleated cells. Cell. 1978;14:843–856. - PubMed
- Brugge J S. Interaction of the Rous sarcoma virus protein pp60src with the cellular proteins pp50 and pp90. Curr Top Microbiol Immunol. 1986;123:1–22. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous