Tenascin-C suppresses Rho activation - PubMed (original) (raw)
Tenascin-C suppresses Rho activation
M B Wenk et al. J Cell Biol. 2000.
Abstract
Cell binding to extracellular matrix (ECM) components changes cytoskeletal organization by the activation of Rho family GTPases. Tenascin-C, a developmentally regulated matrix protein, modulates cellular responses to other matrix proteins, such as fibronectin (FN). Here, we report that tenascin-C markedly altered cell phenotype on a three-dimensional fibrin matrix containing FN, resulting in suppression of actin stress fibers and induction of actin-rich filopodia. This distinct morphology was associated with complete suppression of the activation of RhoA, a small GTPase that induces actin stress fiber formation. Enforced activation of RhoA circumvented the effects of tenascin. Effects of active Rho were reversed by a Rho inhibitor C3 transferase. Suppression of GTPase activation allows tenascin-C expression to act as a regulatory switch to reverse the effects of adhesive proteins on Rho function. This represents a novel paradigm for the regulation of cytoskeletal organization by ECM.
Figures
Figure 1
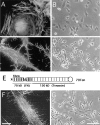
Filopodia form in response to a fibrin-FN+tenascin-C matrix. Fibrin-FN (A and B), fibrin-FN+tenascin-C (C and D), and fibrin-FN+70Ten (F and G) matrices were formed on glass coverslips and cells were allowed to spread for 1 h. Cells were analyzed by phase microscopy or washed, fixed, permeabilized, and incubated with rhodamine-phalloidin to stain filamentous actin. (E) 70Ten contains the amino-terminal 70-kD region of FN including the fibrin cross-linking site (X) connected to all type III repeats and the terminal knob of tenascin-C containing adhesive and anti-adhesive domains. Bars: (A, C, and F) 10 μm; (B, D, and G) 100 μm.
Figure 2
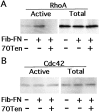
Tenascin-C inhibits Rho activation. Relative amounts of activated (GTP-bound) Rho and Cdc42 were determined using an affinity assay. (A and B) NIH3T3 fibroblasts were serum starved, then plated on tissue culture plastic (−) or on matrices with the indicated components. Active GTPases were isolated and analyzed along side total cell lysates by immunoblotting with anti-Rho or anti-Cdc42 antibodies.
Figure 3
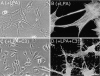
Rho inactivation causes restoration of filopodia. NIH3T3 cells were serum starved for 24 h either without (A and B) or with (C and D) 25 μg/ml C3 transferase added to the medium. Before plating on fibrin-FN+70Ten matrices, all cells were pretreated with 200 ng/ml LPA while in suspension for 30 min. Cells were allowed to spread for 1 h before examination by phase optics (A and C) or visualization of the actin cytoskeleton (B and D). Bars, 20 μm.
Figure 4
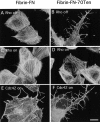
RhoA activity ablates effect of tenascin-C. Rat1 fibroblasts carrying active RhoA-V14 under the control of a tetracycline repressible promoter were allowed to spread on fibrin-FN (A and C) or fibrin-FN+ 70Ten matrix (B and D). Cells under repressed conditions (no active RhoA-V14 protein; A and B) or after induction to express active RhoA-V14 (C and D) were stained with rhodamine-phalloidin. Rat1 cells constitutively expressing active Cdc42-V12 were plated on fibrin-FN (E) or fibrin-FN+ 70Ten (F) matrix for 1 h. Bar, 20 μm.
Figure 5
Matrix induction of filopodia formation. Cells were fixed and stained with rhodamine-phalloidin at the indicated times after plating on fibrin-FN (left) or fibrin-FN+70Ten matrix (right). Bar, 20 μm.
Comment in
- Rho GTPases. Integrating integrin signaling.
Ridley A. Ridley A. J Cell Biol. 2000 Aug 21;150(4):F107-9. doi: 10.1083/jcb.150.4.f107. J Cell Biol. 2000. PMID: 10953018 Free PMC article. No abstract available.
Similar articles
- Tenascin-C modulates matrix contraction via focal adhesion kinase- and Rho-mediated signaling pathways.
Midwood KS, Schwarzbauer JE. Midwood KS, et al. Mol Biol Cell. 2002 Oct;13(10):3601-13. doi: 10.1091/mbc.e02-05-0292. Mol Biol Cell. 2002. PMID: 12388760 Free PMC article. - Role of actin polymerization and adhesion to extracellular matrix in Rac- and Rho-induced cytoskeletal reorganization.
Machesky LM, Hall A. Machesky LM, et al. J Cell Biol. 1997 Aug 25;138(4):913-26. doi: 10.1083/jcb.138.4.913. J Cell Biol. 1997. PMID: 9265656 Free PMC article. - Adenoviral-mediated expression and local deposition of recombinant tenascin-C perturbs cell-dependent matrix contraction.
Hsia HC, Schwarzbauer JE. Hsia HC, et al. J Surg Res. 2006 Nov;136(1):92-7. doi: 10.1016/j.jss.2006.05.030. Epub 2006 Aug 22. J Surg Res. 2006. PMID: 16926030 - Actin cytoskeleton organization in response to integrin-mediated adhesion.
Defilippi P, Olivo C, Venturino M, Dolce L, Silengo L, Tarone G. Defilippi P, et al. Microsc Res Tech. 1999 Oct 1;47(1):67-78. doi: 10.1002/(SICI)1097-0029(19991001)47:1<67::AID-JEMT7>3.0.CO;2-P. Microsc Res Tech. 1999. PMID: 10506763 Review. - Modulation of cell-extracellular matrix interactions.
Sechler JL, Corbett SA, Wenk MB, Schwarzbauer JE. Sechler JL, et al. Ann N Y Acad Sci. 1998 Oct 23;857:143-54. doi: 10.1111/j.1749-6632.1998.tb10114.x. Ann N Y Acad Sci. 1998. PMID: 9917839 Review.
Cited by
- Shaping Oncogenic Microenvironments: Contribution of Fibronectin.
Guerrero-Barberà G, Burday N, Costell M. Guerrero-Barberà G, et al. Front Cell Dev Biol. 2024 Apr 10;12:1363004. doi: 10.3389/fcell.2024.1363004. eCollection 2024. Front Cell Dev Biol. 2024. PMID: 38660622 Free PMC article. Review. - The coordinated activities of collagen VI and XII in maintenance of tissue structure, function and repair: evidence for a physical interaction.
Gregory CA, Ma J, Lomeli S. Gregory CA, et al. Front Mol Biosci. 2024 Mar 28;11:1376091. doi: 10.3389/fmolb.2024.1376091. eCollection 2024. Front Mol Biosci. 2024. PMID: 38606288 Free PMC article. - Biology of Tenascin C and its Role in Physiology and Pathology.
Abedsaeidi M, Hojjati F, Tavassoli A, Sahebkar A. Abedsaeidi M, et al. Curr Med Chem. 2024;31(19):2706-2731. doi: 10.2174/0929867330666230404124229. Curr Med Chem. 2024. PMID: 37021423 Review. - Revisiting the Tenascins: Exploitable as Cancer Targets?
Tucker RP, Degen M. Tucker RP, et al. Front Oncol. 2022 Jun 17;12:908247. doi: 10.3389/fonc.2022.908247. eCollection 2022. Front Oncol. 2022. PMID: 35785162 Free PMC article. Review.
References
- Adams J.C., Watt F.M. Regulation of development and differentiation by the extracellular matrix. Development. 1993;117:1183–1198. - PubMed
- Aukhil I., Joshi P., Yan Y., Erickson H.P. Cell- and heparin-binding domains of the hexabrachion arm identified by tenascin expression proteins. J. Biol. Chem. 1993;268:2542–2553. - PubMed
- Aukhil I., Slemp C.A., Lightner V.A., Nishimura K., Briscoe G., Erickson H.P. Purification of hexabrachion (tenascin) from cell culture conditioned medium and separation from a cell adhesion factor. Matrix. 1990;10:98–111. - PubMed
- Bagrodia S., Derijard B., Davis R.J., Cerione R.A. Cdc42 and PAK-mediated signaling leads to Jun kinase and p38 mitogen-activated protein kinase activation. J. Biol. Chem. 1995;270:27995–27998. - PubMed
- Benard V., Bohl B.P., Bokoch G.M. Characterization of Rac and Cdc42 activation in chemoattractant-stimulated human neutrophils using a novel assay for active GTPases. J. Biol. Chem. 1999;274:13198–13204. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous