KIF5C, a novel neuronal kinesin enriched in motor neurons - PubMed (original) (raw)
KIF5C, a novel neuronal kinesin enriched in motor neurons
Y Kanai et al. J Neurosci. 2000.
Abstract
Kinesin superfamily proteins (KIFs) are the molecular motors conveying cargos along microtubules. KIF5s, the heavy chains of conventional kinesin (KHC), are originally identified members of KIFs, and neuronal KIF5A and ubiquitous KIF5B have been identified so far. In the present work, we cloned a novel member of KIF5, KIF5C, and generated specific antibodies against three KIF5s to investigate their distribution and functions. KIF5A showed pan-neuronal distribution in the nervous system. KIF5B showed a glial cell distribution pattern in general; however, interestingly, its expression was strongly upregulated in axon-elongating neurons, such as olfactory primary neurons and mossy fibers. KIF5C was also a neuronal KIF5 like KIF5A but was highly expressed in lower motor neurons in 2-week-old or older mice, suggesting its important roles in the maintenance of motor neurons rather than in their formation, such as axonal elongation. Because a large part of KIF5s in adult motor neurons were expected to be KIF5C, we generated mice lacking the kif5C gene to investigate the functions of KIF5C in neurons in living animals. The mutant mice showed smaller brain size but were viable and did not show gross changes in the nervous system. Closer examinations revealed the relative loss of motor neurons to sensory neurons. Because three KIF5s showed high similarity in the amino acid sequence, could rescue the KIF5B mutant cells, and could form heterodimers, we think that there are functional redundancy among the three KIF5s and that KIF5A and KIF5B prevented the KIF5C null mice from the severe phenotype.
Figures
Fig. 1.
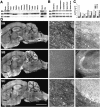
Alignment of the three KIF5s and antibody characterization. A, Alignment of the sequences of the three KIF5s. Amino acids are numbered in the_left margin_. Asterisks represent the identical amino acids among the three KIF5s, and dots_show the same residues between two KIF5s. The motor domain, located in the N-terminal region, is boxed. The amino acid sequences used for the antibody generation are_underlined. B, Homology among the three KIF5s and coiled-coil probability of KIF5C. There were two regions that showed very low similarity among the three KIF5s, with a small coiled-coil probability. We used these sequences for generating antibodies specific for each of the KIF5s (5A-n, 5B-n, 5C-n, 5A-c, 5B-c, and 5C-c). C, Immunoblot of anti-KIF5 antibodies (5A-n, 5B-n, 5C-n, 5A-c, 5B-c, and 5C-c). Ten micrograms of crude extract from an adult mouse brain were loaded on each_lane_. D, Examination of the specificity of the six antibodies using recombinant KIF5 proteins. Filters transferred with 10 μg of crude extract from adult mouse spinal cord (sp) and various amounts of recombinant KIF5 proteins (KIF5A and KIF5C, 1, 3, 10, and 30 ng; KIF5B, 3, 10, 30, and 100 ng) were used. 5A-n showed weak cross-reactivity for KIF5B. 5A-c, 5B-n, 5B-c, and 5C-n did not cross-react with the other members of KIF5. 5C-c was not a specific antibody. E, Immunoblot analysis to characterize the widely used monoclonal anti-kinesin antibodies (SUK4, H1, and H2), using equal amount of recombinant KIF5 proteins (10 ng for SUK4 and H2; 30 ng for H1). SUK4 predominantly stained KIF5A; H1 detected only KIF5C; H2 preferred KIF5A and KIF5C to KIF5B. H1 did not recognize KIF5A or KIF5B, even when 100 ng of proteins were loaded (data not shown).
Fig. 2.
KIF5A and KIF5C are neuronal kinesins but showed differential distribution in the nervous system.A, Left, Tissue distribution of the three KIF5s by immunoblotting. KIF5B was detected in every tissue investigated, whereas KIF5A and KIF5C were expressed exclusively in the tissues of nervous system (Brain, Spinal Cord). A, Right, Immunoblot of cultured hippocampal neurons (Neuron) and glial cells (Glia). KIF5B was expressed in both neurons and glial cells, whereas the expression of KIF5A and KIF5C was limited to neurons and was not found in glial cells. B, C, Expression of the three KIF5s in nervous system as determined by quantitative immunoblotting. The olfactory bulb, cortex, hippocampus, cerebellum, spinal cord, and sciatic nerve were investigated. KIF5B was the most abundantly expressed KIF5 throughout the nervous system (70–90% of the total KIF5). As for the neuronal KIF5s, the expression ratio of KIF5C to KIF5A varied from one to five among different regions of the nervous system. Note the prominent expression of KIF5B in the olfactory bulb. D, Distribution of each of the KIF5s in the brain as shown by immunofluorescence analysis using anti-KIF5 antibodies (left, low magnification;middle and right, high magnification of the boxed areas in the left panels).5A, KIF5A stained various kinds of neurons. It was found in cell bodies, dendrites, and axons. Arrowheads in the_right panel_ indicate the axons in the anterior funiculus of the spinal cord. 5B, Although KIF5B was highly expressed in the mossy fibers of the hippocampus (arrowheads in the right panel), anti-KIF5B antibody primary stained glial cells (e.g., astroglias in cortex; middle panel). 5C, KIF5C was expressed only in the subpopulation of neurons, especially in medulla (middle panel) and spinal cord (right panel). In spinal cord, KIF5C was highly expressed in the neurons located at the anterior horn (arrowheads). Note the tendency of a similar staining pattern in the cortex, hippocampus, cerebellum, and spinal cord to the immunoblotting data. Scale bars: left, 5 mm;middle, right, 0.5 mm.
Fig. 3.
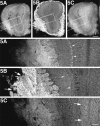
KIF5B is highly expressed in olfactory primary axons. Immunofluorescence of the olfactory bulb after staining with anti-KIF5 antibodies. The bottom panels represent high magnifications of the boxed areas of the top panels, respectively. KIF5B was highly expressed in the olfactory primary axons (arrows in 5B); however, glial cells, mainly astrocytes (arrowheads in_5B_), were stained predominantly in the rest of the olfactory bulb. On the other hand, KIF5A and KIF5C were localized only to the neuronal cells (e.g., mitral cell; arrowheads in_5A_). Note the strong staining of KIF5C in the granule cells (arrows in 5C). Scale bars:top, 0.4 mm (low magnifications); bottom, 0.1 mm (high magnifications).
Fig. 4.
KIF5C is highly expressed in motor neurons. Localization of the three KIF5s in the brain and spinal cord.a–i, Immunofluorescence of brain frontal sections including the abducens nucleus (VI) [low magnification (a, d, g) and high magnification (b, e, h) of the boxed areas_] and cross-sections of the spinal cord at the C7 level (c, f, i) after staining with anti-KIF5 antibodies (KIF5A, a–c; KIF5B, d–f; KIF5C, g–i). KIF5B was highly expressed in glial cells [mainly astrocytes (brain) and oligodendrocytes (spinal cord)] but was less abundant in neurons. KIF5A and KIF5C were localized to neurons but showed different expression patterns. KIF5A was found at similar levels of expression in various kinds of neurons, whereas KIF5C was highly expressed in motor neurons both in the brain (VI) and in the spinal cord (MN). j–s, Distribution of KIF5A (j–n) and KIF5C (o–s) in the sections containing several cranial nerves. KIF5A was expressed equally among neurons as described above. However, prominent expression of KIF5C was observed in motor [oculomotor (III), motor trigeminal (MoV), and facial (VII) nerves], but the expression was weaker in the sensory [mesencephalic trigeminal (MeV) and vestibulocochlear (VIII)] neurons. k,l, n, p, q, and s represent higher magnifications of the_boxed areas in their left panels, respectively. Note the higher expression of KIF5A in sensory (MeV) than in motor (MoV) neurons, in contrast to KIF5C. Arrows and_arrowheads_ indicate cell bodies and axons, respectively. Scale bars: a, d, g,j, m, o, r, 1 mm (low magnifications); b, c,e, f, h, i,k, l, n, p,q, s, 0.4 mm (high magnifications).
Fig. 5.
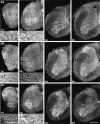
KIF5C is increased developmentally in motor neurons. Distribution of the three KIF5s in the mouse spinal cords 1, 2, 4, and 8 weeks after birth. Bottom panels of the spinal cords at 1 or 2 weeks old are the high magnifications of the_boxed areas_ of the top panels. KIF5A showed pan-neuronal distribution (arrows in_5A_), and KIF5B was expressed dominantly in glial cells (arrowheads in 5B; radial glias are oligodendroglias) throughout this period. However, the expression pattern of KIF5C changed developmentally; the prominent expression of KIF5C in motor neurons was not found in 1-week-old mice but was observed in 2-week-old or older mice, which was increased developmentally (arrows in 5C). Scale bars: bottom left, 0.4 mm (low magnifications);bottom right, 0.1 mm (high magnifications).
Fig. 6.
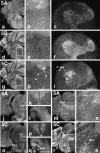
Targeted disruption of the mouse_kif5C_ gene. A, Targeting strategy with conventional positive-negative selection. The strategy of genomic Southern blotting for the screening of homologous recombinant ES clones is included. SA, Splicing acceptor; filled box, exon including ATP-binding motif (P-loop);Sp, _Spe_I; RV,_Eco_RV; Sal, _Sal_I;H, _Hin_dIII; Nc,_Nco_I; Xb, Xba_I.B, Southern blotting analysis of targeted ES clones digested, respectively, by Spe_I and_Eco_RV. Clones D8 and L8 were homologous recombinants.Con was a nonhomologous control. C, Comparison of body and brain size among adult kif5C+/+,+/−, and −/− mice. Although the body size of null mutants was not significantly altered, they showed smaller brains than wild-type and heterozygote mice (∼5% decrease in weight). Scale bar, 5 mm. D, E, Northern blotting and immunoblotting analysis of KIF5C knock-out mice. Total brain RNAs and brain crude extracts were prepared from adult mice. The total RNAs were hybridized, respectively, with probes for KIF5C and β-actin (D), and the crude extracts (10 μg) were blotted using anti-KIF5 antibodies (E). In both cases, no KIF5C was detected in the KIF5C null mice. As for the effects of the absence of KIF5C on the other KIF5s, KIF5A and KIF5B showed little change in their expression levels in the brains of knock-out mice. F, Immunofluorescence analysis of KIF5C knock-out mice. Spinal cords of the wild-type and KIF5C null mutants were cross-sectioned at the C7 level and stained with anti-KIF5 antibodies. The staining pattern of the wild-type mouse spinal cord was shown previously in Figure 4,c, f, and i. KIF5A and KIF5B showed similar expression pattern as in the wild-type mice. Staining intensity of the anti-KIF5C antibody in the null mutants was at a background level. Scale bar, 0.4 mm. G, Bodian staining of frontal sections of the wild-type (+/+) and mutant (−/−) mice. The panels on the right represent high magnifications of the boxed areas. The brains of mutant mice were smaller but did not show any gross abnormality in their structures. Scale bars: left, 1 mm;right, 0.2 mm. H, Comparison of motor neurons (VI, abducens nucleus) between the wild-type (+/+) and mutant (−/−) mice 16 weeks of age using serial sections. The number of neurons in the abducens nucleus in the mutant mice (236 ± 14) was decreased to 72 ± 5% of that in the wild-type mice (326 ± 12) (n = 4). Scale bar, 0.1 mm. I, Comparison of sensory neurons in the sensory trigeminal neurons between wild-type (+/+) and mutant (−/−) 8-week-old mice (arrows). The numbers of sensory neurons in the wild-type and mutant mice were 398 ± 23 and 439 ± 31, respectively. Sensory trigeminal neurons did not decrease but rather showed the tendency of slight increase (110 ± 9%,n = 6). Scale bar, 0.2 mm. J, Developmental comparison of motor neurons (VI) between the wild-type and mutant mice (1-, 2-, 4-, 8-, and 16-week-old). The number of neurons in the abducens nucleus in the mutant mice showed little difference from that in the wild-type 1-week-old mice (98 ± 5%) but was decreased developmentally in those 2 weeks or later after birth (2 weeks, 88 ± 5% to 16 weeks, 72 ± 5%).
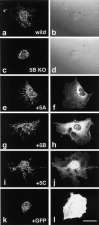
Fig. 7.
Rescue of abnormal mitochondrial localization in the KIF5B null mutant cells by each of the KIF5s.a, b, Wild-type control.c–l, KIF5B null mutant cells. Cells were injected with the expression vectors of KIF5A (e,f), KIF5B (g,h), KIF5C (i, j), or GFP (k, l). Mitochondrial staining using Mitotracker (a, c,e, g, i,k), Nomarski images (b,d), immunofluorescence after staining with each of the KIF5s (f, h, j), and fluorescence by exogenous GFP (l). Note the recovery of mitochondrial dispersion radiating from the cell center as in wild-type cells after microinjection of KIF5 but not after that of GFP plasmids. Scale bar, 50 μm.
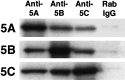
Fig. 8.
Coimmunoprecipitation of three KIF5s with each of the anti-KIF5 antibodies. Mouse brain crude extracts were immunoprecipitated separately by anti-KIF5 antibodies or normal rabbit IgG (_Anti_-5A, Anti-5B,Anti-5C, and Rab IgG, respectively) and then blotted with each of the anti-KIF5 antibodies (5A,5B, and 5C, respectively). The sample precipitated by antibodies directed against one KIF5 also included the other KIF5s. The control normal rabbit IgG did not precipitate any KIF5.
Similar articles
- Association of the kinesin-binding domain of RanBP2 to KIF5B and KIF5C determines mitochondria localization and function.
Cho KI, Cai Y, Yi H, Yeh A, Aslanukov A, Ferreira PA. Cho KI, et al. Traffic. 2007 Dec;8(12):1722-1735. doi: 10.1111/j.1600-0854.2007.00647.x. Epub 2007 Sep 21. Traffic. 2007. PMID: 17887960 - Specific depletion of the motor protein KIF5B leads to deficits in dendritic transport, synaptic plasticity and memory.
Zhao J, Fok AHK, Fan R, Kwan PY, Chan HL, Lo LH, Chan YS, Yung WH, Huang J, Lai CSW, Lai KO. Zhao J, et al. Elife. 2020 Jan 21;9:e53456. doi: 10.7554/eLife.53456. Elife. 2020. PMID: 31961321 Free PMC article. - Temporal and tissue specific gene expression patterns of the zebrafish kinesin-1 heavy chain family, kif5s, during development.
Campbell PD, Marlow FL. Campbell PD, et al. Gene Expr Patterns. 2013 Oct;13(7):271-9. doi: 10.1016/j.gep.2013.05.002. Epub 2013 May 15. Gene Expr Patterns. 2013. PMID: 23684767 Free PMC article. - Role of kinesin-1 in the pathogenesis of SPG10, a rare form of hereditary spastic paraplegia.
Kawaguchi K. Kawaguchi K. Neuroscientist. 2013 Aug;19(4):336-44. doi: 10.1177/1073858412451655. Epub 2012 Jul 10. Neuroscientist. 2013. PMID: 22785106 Review. - Understanding how kinesin motor proteins regulate postsynaptic function in neuron.
Fan R, Lai KO. Fan R, et al. FEBS J. 2022 Apr;289(8):2128-2144. doi: 10.1111/febs.16285. Epub 2021 Dec 3. FEBS J. 2022. PMID: 34796656 Review.
Cited by
- Normal levels of KIF5 but reduced KLC1 levels in both Alzheimer disease and Alzheimer disease in Down syndrome: evidence suggesting defects in anterograde transport.
Chen XQ, Das U, Park G, Mobley WC. Chen XQ, et al. Alzheimers Res Ther. 2021 Mar 10;13(1):59. doi: 10.1186/s13195-021-00796-6. Alzheimers Res Ther. 2021. PMID: 33691783 Free PMC article. - Mitochondrial transport in neurons: impact on synaptic homeostasis and neurodegeneration.
Sheng ZH, Cai Q. Sheng ZH, et al. Nat Rev Neurosci. 2012 Jan 5;13(2):77-93. doi: 10.1038/nrn3156. Nat Rev Neurosci. 2012. PMID: 22218207 Free PMC article. Review. - In vitro selection of Jun-associated proteins using mRNA display.
Horisawa K, Tateyama S, Ishizaka M, Matsumura N, Takashima H, Miyamoto-Sato E, Doi N, Yanagawa H. Horisawa K, et al. Nucleic Acids Res. 2004 Dec 2;32(21):e169. doi: 10.1093/nar/gnh167. Nucleic Acids Res. 2004. PMID: 15576676 Free PMC article. - Mitochondrial heterogeneity and homeostasis through the lens of a neuron.
Pekkurnaz G, Wang X. Pekkurnaz G, et al. Nat Metab. 2022 Jul;4(7):802-812. doi: 10.1038/s42255-022-00594-w. Epub 2022 Jul 11. Nat Metab. 2022. PMID: 35817853 Free PMC article. Review. - Network-centric medicine for peripheral nerve injury: Treating the whole to boost endogenous mechanisms of neuroprotection and regeneration.
Romeo-Guitart D, Casas C. Romeo-Guitart D, et al. Neural Regen Res. 2019 Jul;14(7):1122-1128. doi: 10.4103/1673-5374.251187. Neural Regen Res. 2019. PMID: 30804234 Free PMC article.
References
- Beushausen S, Kladakis A, Jaffe H. Kinesin light chains: identification and characterization of a family of proteins from the optic lobe of the squid Loligo pealii. DNA Cell Biol. 1993;12:901–909. - PubMed
- Bodian P. A new method for staining nerve fibers and nerve ending in mounted paraffin sections. Anat Rec. 1936;65:89–96.
- Bost-Usinger L, Chen RJ, Hillman D, Park H, Burnside B. Multiple kinesin family members expressed in teleost retina and RPE include a novel C-terminal kinesin. Exp Eye Res. 1997;64:781–794. - PubMed
- Brady ST. A novel brain ATPase with properties expected for the fast axonal transport motor. Nature. 1985;317:73–75. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous