p53 transcriptional activity is essential for p53-dependent apoptosis following DNA damage - PubMed (original) (raw)
p53 transcriptional activity is essential for p53-dependent apoptosis following DNA damage
C Chao et al. EMBO J. 2000.
Abstract
p53-mediated transcription activity is essential for cell cycle arrest, but its importance for apoptosis remains controversial. To address this question, we employed homologous recombination and LoxP/Cre-mediated deletion to produce mutant murine embryonic stem (ES) cells that express p53 with Gln and Ser in place of Leu25 and Trp26, respectively. p53(Gln25Ser26) was stable but did not accumulate after DNA damage; the expression of p21/Waf1 and PERP was not induced, and p53-dependent repression of MAP4 expression was abolished. Therefore, p53(Gln25Ser26) is completely deficient in transcriptional activation and repression activities. After DNA damage by UV radiation, p53(Gln25Ser26) was phosphorylated at Ser18 but was not acetylated at C-terminal sites, and its DNA binding activity did not increase, further supporting a role for p53 acetylation in the activation of sequence-specific DNA binding activity. Most importantly, p53(Gln25Ser26) mouse thymocytes and ES cells, like p53(-/-) cells, did not undergo DNA damage-induced apoptosis. We conclude that the transcriptional activities of p53 are required for p53-dependent apoptosis.
Figures
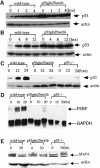
Fig. 1. Construction of p53Gln25Ser26 ES cells. (A) The mouse germline p53 locus. Blank boxes represent the p53 exons and the two filled bars represent the two probes (A and B) used to detect the wild-type and mutant p53 alleles by Southern blot analysis. The germline 14 kb _Eco_RI and 5.6 kb _Bam_HI fragments are indicated. (B) The targeting construct. The position of the mutations encoding Gln25 and Ser26 in place of Leu25 and Trp26 in exon 2 of the p53 gene is indicated by an asterisk. The _PGK-Neo_r gene flanked by LoxP sites was inserted into an engineered _Sal_I site within intron 4. (C) Targeted p53 locus. The sizes of the mutant _Eco_RI and _Bam_HI fragments are indicated. The positions of the PCR primer sites that were used to screen for deletion of the _PGK-Neo_r segment are indicated by arrowheads. (D) Mutant p53 allele with the _PGK-Neo_r gene segment deleted. The size of the mutant _Bam_HI fragment after deletion of the _PGK-Neo_r gene is indicated; arrows show the new positions of the PCR primer sites. (E) Southern blot analysis of genomic DNA derived from wild-type (lane 1), heterozygous p53Gln25Ser26 mutant (lanes 2 and 3) and homozygous mutant (lanes 4 and 5) ES cells with the _PGK-Neo_r gene inserted. Genomic DNA was digested with _Eco_RI and hybridized with probe A. The positions of the _Eco_RI restriction fragments from the germline alleles are indicated by an arrow. (F) Southern blotting analysis of genomic DNA derived from wild-type (lane 1), homozygous mutant ES cells with the _PGK-Neo_r gene inserted (lane 2), and p53Gln25Ser26 ES cells with the _PGK-Neo_r gene deleted (lanes 3 and 4). Genomic DNA was digested with _Bam_HI and hybridized with probe B. The positions of the _Bam_HI fragments derived from wild-type and mutant alleles after deletion of the _PGK-Neo_r gene and of the mutant allele with the _PGK-Neo_r gene inserted are indicated with arrows.
Fig. 2. Induction of p53 and p53-dependent gene expression in wild-type and p53Gln25Ser26 cells following DNA damage. Cell extracts were prepared from wild-type and p53Gln25Ser26 ES cells at the time points indicated after exposure to (A) 5 Gy γ-irradiation or (B) 60 J/m2 UV-C light, and the samples were processed for western immunoblot analysis as described in Materials and methods. The genotypes and time points are labeled on the top of the lane. p53 and actin are indicated on the right. (C) p21 protein induction in wild-type, p53Gln25Ser26 and p53–/– differentiated ES cells following 60 J/m2 UV treatment. The positions of p21 and actin are indicated by arrows. (D) PERP mRNA induction in wild-type, p53Gln25Ser26 and p53–/– differentiated ES cells following 60 J/m2 UV treatment. The positions of PERP and GAPDH mRNA are indicated by arrows. (E) Repression of MAP4 expression in wild-type, p53Gln25Ser26 and p53–/– ES cells following 60 J/m2 UV radiation. The positions of MAP4 protein and actin are indicated with arrows. The genotypes and times after DNA damage are given at the top of each panel.
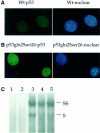
Fig. 3. Cellular localization and DNA binding of p53Gln25Ser26. Indirect immunofluorescence: wild-type (A) and p53Gln25Ser26 (B) differentiated ES cells were fixed and stained with PAb421 for p53 (left panels) and DAPI for DNA (right panels) as described in Materials and methods. (C) EMSA: nuclear extracts were prepared from wild-type, p53Gln25Ser26 and p53–/– differentiated ES cells before or 4 h after exposure to 60 J/m2 UV light. EMSA was performed using a 32P-labeled, double-stranded p53 consensus binding sequence as described in Materials and methods. Shown is an autoradiogram of the polyacrylamide gel. The lanes correspond to the nuclear p53-specific DNA binding activity from: 1, p53–/– differentiated ES cells; 2, wild-type differentiated ES cells with no treatment; 3, wild-type differentiated ES cells 4 h after exposure to UV; 4, differentiated p53Gln25Ser26 ES cells with no UV treatment; 5, differentiated p53Gln25Ser26 ES cells 4 h after exposure to UV. The positions of supershifted (SS) as well as specific (S) p53-specific DNA complexes are indicated. PAb421 antibody against p53 was used to supershift the p53 complexes.
Fig. 4. UV-induced phosphorylation and acetylation of p53 in wild-type and p53Gln25Ser26 cells. (A) Western immunoblot analysis showing the phosphorylation of mouse p53 at Ser18 in wild-type and p53Gln25Ser26 ES cells before and 2 or 4 h after exposure to 60 J/m2 UV light. The immunoblot was probed with affinity purified antibodies specific for murine p53 phosphorylated at Ser18 (p53-Ser18P, top strip); the blot was then stripped and probed with PAb240 to detect the total p53 signal (bottom strip). (B) Western immunoblot showing the acetylation of mouse p53 at Lys317 and Lys379 (corresponding to human Lys320 and Lys382) in differentiated wild-type and p53Gln25Ser26 ES cells before and 18 or 24 h after exposure to 60 J/m2 UV light. The blot was probed with affinity purified antibodies specific for mouse p53 acetylated at Lys317 (corresponding to Lys320 of human p53; top strip), stripped and reprobed with antibodies specific for mouse p53 acetylated at Lys379 (corresponding to Lys382 of human p53; central strip), and finally stripped and reprobed with PAb240. The genotypes are given above the panels, the positions of p53 are indicated with arrows.
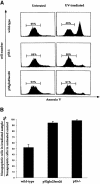
Fig. 5. Induction of apoptosis in wild-type, p53–/– and p53Gln25Ser26 ES cells by UV treatment. (A) Flow cytometric analysis of wild-type, p53–/– and p53Gln25Ser26 ES cells harvested 12 h after exposure to 60 J/m2 UV irradiation. Cell number is plotted as a function of the intensity of staining for annexin V; cells stained positive with annexin V antibodies are apoptotic. The percentages of non-apoptotic cells are indicated. (B) The percentile ratio of non-apoptotic cells in irradiated wild-type, p53Gln25Ser26 and p53–/– ES cells relative to non-apoptotic cells in unirradiated controls. The mean and standard deviation from three independent experiments is given.
Fig. 6. Induction of apoptosis in wild-type, p53Gln25Ser26 and p53–/– thymocytes by ionizing radiation. (A) Thymocytes were harvested from wild-type, p53–/– mice and p53Gln25Ser26–RAG2–/– chimeric mice, stained for CD4 and CD8, and analyzed by flow cytometry as described in Materials and methods. Cells residing in the lymphocyte gate were analyzed and the percentage of total cells in a particular gate is indicated. (B) The mean value of the percentile ratio of non-apoptotic CD4+ thymocyte number in wild-type, p53Gln25Ser26 and p53–/– thymocytes treated with 5, 10 and 20 Gy of IR to the non-apoptotic thymocyte number from untreated controls from three independent experiments is given. Error bars show the standard deviation.
Similar articles
- Cell type- and promoter-specific roles of Ser18 phosphorylation in regulating p53 responses.
Chao C, Hergenhahn M, Kaeser MD, Wu Z, Saito S, Iggo R, Hollstein M, Appella E, Xu Y. Chao C, et al. J Biol Chem. 2003 Oct 17;278(42):41028-33. doi: 10.1074/jbc.M306938200. Epub 2003 Aug 8. J Biol Chem. 2003. PMID: 12909629 - The apoptotic and transcriptional transactivation activities of p53 can be dissociated.
Bissonnette N, Wasylyk B, Hunting DJ. Bissonnette N, et al. Biochem Cell Biol. 1997;75(4):351-8. Biochem Cell Biol. 1997. PMID: 9493957 - Ets1 is required for p53 transcriptional activity in UV-induced apoptosis in embryonic stem cells.
Xu D, Wilson TJ, Chan D, De Luca E, Zhou J, Hertzog PJ, Kola I. Xu D, et al. EMBO J. 2002 Aug 1;21(15):4081-93. doi: 10.1093/emboj/cdf413. EMBO J. 2002. PMID: 12145208 Free PMC article. - Mechanisms of p53-induced apoptosis: in search of genes which are regulated during p53-mediated cell death.
Choisy-Rossi C, Reisdorf P, Yonish-Rouach E. Choisy-Rossi C, et al. Toxicol Lett. 1998 Dec 28;102-103:491-6. doi: 10.1016/s0378-4274(98)00238-0. Toxicol Lett. 1998. PMID: 10022301 Review. - Regulation of p53 downstream genes.
el-Deiry WS. el-Deiry WS. Semin Cancer Biol. 1998;8(5):345-57. doi: 10.1006/scbi.1998.0097. Semin Cancer Biol. 1998. PMID: 10101800 Review.
Cited by
- p53 regulates diverse tissue-specific outcomes to endogenous DNA damage in mice.
Hill RJ, Bona N, Smink J, Webb HK, Crisp A, Garaycoechea JI, Crossan GP. Hill RJ, et al. Nat Commun. 2024 Mar 21;15(1):2518. doi: 10.1038/s41467-024-46844-1. Nat Commun. 2024. PMID: 38514641 Free PMC article. - BNIP3-dependent mitophagy safeguards ESC genomic integrity via preventing oxidative stress-induced DNA damage and protecting homologous recombination.
Zhao Q, Liu K, Zhang L, Li Z, Wang L, Cao J, Xu Y, Zheng A, Chen Q, Zhao T. Zhao Q, et al. Cell Death Dis. 2022 Nov 19;13(11):976. doi: 10.1038/s41419-022-05413-4. Cell Death Dis. 2022. PMID: 36402748 Free PMC article. - A pan-cancer analysis of the oncogenic role of dual-specificity tyrosine (Y)-phosphorylation- regulated kinase 2 (DYRK2) in human tumors.
Qiu X, Shen C, Zhao W, Zhang X, Zhao D, Wu X, Yang L. Qiu X, et al. Sci Rep. 2022 Sep 14;12(1):15419. doi: 10.1038/s41598-022-19087-7. Sci Rep. 2022. PMID: 36104345 Free PMC article. - Sensitivity to tumor development by TALEN-mediated Trp53 mutant genes in the susceptible FVB/N mice and the resistance C57BL/6 mice.
Yun WB, Kim JE, Lee ML, Choi JY, Park JJ, Song BR, Kang BC, Nam KT, Lee HW, Hwang DY. Yun WB, et al. Lab Anim Res. 2021 Nov 29;37(1):32. doi: 10.1186/s42826-021-00107-y. Lab Anim Res. 2021. PMID: 34839833 Free PMC article. - Investigation of the Crosstalk between GRP78/PERK/ATF-4 Signaling Pathway and Renal Apoptosis Induced by Nephropathogenic Infectious Bronchitis Virus Infection.
Chen W, Huang C, Shi Y, Li N, Wang E, Hu R, Li G, Yang F, Zhuang Y, Liu P, Hu G, Gao X, Guo X. Chen W, et al. J Virol. 2022 Jan 26;96(2):e0142921. doi: 10.1128/JVI.01429-21. Epub 2021 Oct 20. J Virol. 2022. PMID: 34669445 Free PMC article.
References
- Aladjem M.I., Spike,B.T., Rodewald,L.W., Hope,T.J., Klemm,M., Jaenisch,R. and Wahl,G.M. (1998) ES cells do not activate p53-dependent stress responses and undergo p53-independent apoptosis in response to DNA damage. Curr. Biol., 8, 145–155. - PubMed
- Banin S. et al. (1998) Enhanced phosphorylation of p53 by ATM in response to DNA damage. Science, 281, 1674–1677. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous