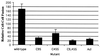Critical role for the cysteines flanking the internal fusion peptide of avian sarcoma/leukosis virus envelope glycoprotein - PubMed (original) (raw)
Critical role for the cysteines flanking the internal fusion peptide of avian sarcoma/leukosis virus envelope glycoprotein
S E Delos et al. J Virol. 2000 Oct.
Abstract
The transmembrane subunit (TM) of the envelope glycoprotein (Env) of the oncovirus avian sarcoma/leukosis virus (ASLV) contains an internal fusion peptide flanked by two cysteines (C9 and C45). These cysteines, as well as an analogous pair in the Ebola virus GP glycoprotein, are predicted to be joined by a disulfide bond. To examine the importance of these cysteines, we mutated C9 and C45 in the ASLV subtype A Env (EnvA), individually and together, to serine. All of the mutant EnvAs formed trimers that were composed of the proteolytically processed surface (SU) and TM subunits. All mutant EnvAs were incorporated into murine leukemia virus pseudotyped virions and bound receptor with wild-type affinity. Nonetheless, all mutant EnvAs were significantly impaired ( approximately 1,000-fold) in their ability to support infectivity. They were also significantly impaired in their ability to mediate cell-cell fusion. Our data are consistent with a model in which the internal fusion peptide of ASLV-A EnvA exists as a loop that is stabilized by a disulfide bond at its base and in which this stabilized loop serves an important function during virus-cell fusion. The fusion peptide of the Ebola virus GP glycoprotein may conform to a similar structure.
Figures
FIG. 1
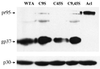
Trimerization of mutant EnvAs. 293T cells were transfected with pCB6-EnvA DNA by the calcium phosphate method and induced with 10 mM sodium butyrate 24 h later. Sixteen to 18 h posttransfection, cells were harvested, lysed in 1% NP-40 buffer containing protease inhibitors (3), and subjected to sucrose density centrifugation in 10 to 30% sucrose in _n_-octyl glucoside buffer as described previously (3). Fractions (500 μl each) were collected and processed for Western blot analysis with the anti-Ngp37 antibody which recognizes both TM and the full-length EnvA precursor, pr95 (10). The gp37 band is shown. The gradient density increases from left to right. C9S, C45S, and C9,45S contain serines in place of the cysteine at the numbered site(s); numbering is from the initial residue of the mature TM subunit. The numbered lanes contain samples from the specified gradient fractions. B denotes a sample from the bottom of the centrifuge tube. WTA, wild-type EnvA.
FIG. 2
Processing of mutant EnvAs. Cell lysates were prepared and processed for Western blot analysis as described in the legend to Fig. 1. Both the uncleaved pr95 (top) and cleaved gp37 (bottom) are indicated. Samples are labeled as in the legend to Fig. 1. Acl is described in Table 1, footnote b.
FIG. 3
Incorporation of mutant EnvAs into MLV pseudotyped virions. EnvA-MLV pseudotyped virions were prepared and concentrated as previously described (3). Virions were diluted into sodium dodecyl sulfate sample buffer, resolved by sodium dodecyl sulfate-polyacrylamide gel electrophoresis, transferred to nitrocellulose, and probed with the anti-Ngp37 antibody. Parallel blots were probed with an antibody recognizing the MLV capsid (p30). Samples are labeled as described in the legend to Fig. 1.
FIG. 4
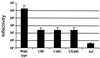
Infectivity of MLV pseudotyped mutant EnvA viruses. EnvA pseudotyped viruses were prepared and titered on PG950 cells as described previously (3). Results are an average of three independent experiments. Samples are labeled as in the legend to Fig. 1. Wild-type, wild-type EnvA.
FIG. 5
Cell-cell fusion mediated by mutant EnvAs. Cell-cell fusion assays were performed as described previously (11), except that modified vaccinia virus Ankara-infected PG950 cells were incubated overnight in medium containing rifampin (100 μg/ml), and the cell-cell fusion medium contained both rifampin (100 μg/ml) and AraC (150 μg/ml). It should be noted that at no time did the pH of any of these media dip below pH 7.2. The data from triplicate samples from a representative experiment were averaged. Samples are labeled as in the legend to Fig. 1. Wild-type, wild-type EnvA.
FIG. 6
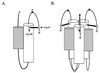
Model of a fusion-active state of the ASLV TM trimer. The core monomer and six-helix bundle formed by the gp37 trimer were modeled after the crystal structures of the core fragment of Ebola virus GP2 (13a, 15). (A) The gp37 monomer. The predicted N-terminal (white) and C-terminal (gray) α-helices pack against each other in an antiparallel orientation, placing the N-terminal loop (containing the fusion peptide) and the C-terminal portions of the protein near each other, extending toward the common fused membrane. A star at the apex of the loop (P) denotes the predicted position of the proline within the fusion peptide. The loop containing the proline is shown stabilized by a disulfide bond between C9 (Cys9) and C45 (Cys45). The disulfide-bonded cysteines are represented by a dumbbell and marked by arrows. The N and C termini are marked. (B) The gp37 trimer. By analogy to other trimeric viral fusion proteins, the N-terminal helices are expected to pack against each other, forming a threefold symmetry axis down the center of the resulting coil (13a, 15) with a given C-terminal helix resting in the groove made by its own and a neighboring N-terminal helix. The C-terminal residues extending from these helices have been omitted for clarity.
Similar articles
- The central proline of an internal viral fusion peptide serves two important roles.
Delos SE, Gilbert JM, White JM. Delos SE, et al. J Virol. 2000 Feb;74(4):1686-93. doi: 10.1128/jvi.74.4.1686-1693.2000. J Virol. 2000. PMID: 10644338 Free PMC article. - Alpharetrovirus envelope-receptor interactions.
Barnard RJ, Young JA. Barnard RJ, et al. Curr Top Microbiol Immunol. 2003;281:107-36. doi: 10.1007/978-3-642-19012-4_3. Curr Top Microbiol Immunol. 2003. PMID: 12932076 Review. - Avian sarcoma and leukosis virus-receptor interactions: from classical genetics to novel insights into virus-cell membrane fusion.
Barnard RJ, Elleder D, Young JA. Barnard RJ, et al. Virology. 2006 Jan 5;344(1):25-9. doi: 10.1016/j.virol.2005.09.021. Virology. 2006. PMID: 16364732 Review.
Cited by
- A conserved sequence within the H2 subunit of the vaccinia virus entry/fusion complex is important for interaction with the A28 subunit and infectivity.
Nelson GE, Wagenaar TR, Moss B. Nelson GE, et al. J Virol. 2008 Jul;82(13):6244-50. doi: 10.1128/JVI.00434-08. Epub 2008 Apr 16. J Virol. 2008. PMID: 18417576 Free PMC article. - The hr1 and fusion peptide regions of the subgroup B avian sarcoma and leukosis virus envelope glycoprotein influence low pH-dependent membrane fusion.
Babel AR, Bruce J, Young JA. Babel AR, et al. PLoS One. 2007 Jan 24;2(1):e171. doi: 10.1371/journal.pone.0000171. PLoS One. 2007. PMID: 17245447 Free PMC article. - Mutational evidence for an internal fusion peptide in flavivirus envelope protein E.
Allison SL, Schalich J, Stiasny K, Mandl CW, Heinz FX. Allison SL, et al. J Virol. 2001 May;75(9):4268-75. doi: 10.1128/JVI.75.9.4268-4275.2001. J Virol. 2001. PMID: 11287576 Free PMC article. - Sequential roles of receptor binding and low pH in forming prehairpin and hairpin conformations of a retroviral envelope glycoprotein.
Matsuyama S, Delos SE, White JM. Matsuyama S, et al. J Virol. 2004 Aug;78(15):8201-9. doi: 10.1128/JVI.78.15.8201-8209.2004. J Virol. 2004. PMID: 15254191 Free PMC article.
References
- Durell S R, Martin I, Ruysschaert J M, Shai Y, Blumenthal R. What studies of fusion peptides tell us about viral envelope glycoprotein-mediated membrane fusion. Mol Membr Biol. 1997;14:97–112. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous