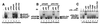Selective requirement for c-Rel during IL-12 P40 gene induction in macrophages - PubMed (original) (raw)
Selective requirement for c-Rel during IL-12 P40 gene induction in macrophages
S Sanjabi et al. Proc Natl Acad Sci U S A. 2000.
Abstract
A major challenge in the study of gene regulation by NF-kappaB/Rel transcription factors is to understand, at the biological and mechanistic levels, the selective functions of individual Rel family members. To study selectivity, we have examined the NF-kappaB/Rel protein binding site (Rel site) within the IL-12 p40 promoter. IL-12 is a proinflammatory cytokine expressed by activated macrophages that serves as an essential inducer of T helper 1 cell development. In nuclear extracts from lipopolysaccharideactivated macrophages, the predominant Rel dimers capable of binding the IL-12 p40 Rel site were the p50/p65 and p50/c-Rel heterodimers and p50/p50 homodimer. The two heterodimers bound the site with comparable affinities and exhibited comparable transactivation activities. In striking contrast, p40 mRNA and protein concentrations were reduced dramatically in c-Rel(-/-) macrophages and only modestly in p65(-/-) macrophages. Other proinflammatory cytokine mRNAs and proteins were not significantly reduced in c-Rel(-/-) macrophages. These results reveal that a c-Rel-containing complex is an essential and selective activator of p40 transcription, which may reflect unique regulatory mechanisms or biological functions of IL-12. Furthermore, because selectivity was not observed in vitro or in transient transactivation experiments, these findings suggest that an understanding of the selectivity mechanism may require an analysis of the endogenous p40 locus.
Figures
Figure 1
Three Rel dimers bind the p40 Rel site in vitro. (A) Gel shift assays were performed with nuclear extracts (4 μg) from 293T cells containing overexpressed Rel proteins as indicated. (B) Gel shift assays were performed with extracts containing overexpressed p50/p65 (lanes 1–4) and p50/c-Rel (lanes 5–8). Specific antibodies were included as indicated. (C) Gel shift assays were performed with extracts (8 μg) from unactivated (lane 1) or LPS/IFN-γ-activated (lanes 2–8) J774 cells.
Figure 2
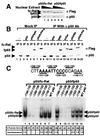
p50/p65 and p50/c-Rel bind the p40 Rel site with comparable affinities. (A) 293T-cell extracts were prepared containing similar concentrations of p50/fc-Rel and p50/fp65. Three concentrations of each extract were analyzed by Western blot, using Flag and p50 antibodies. (B) The extracts were analyzed by immunoprecipitation to determine the fraction of fc-Rel and fp65 associated with p50. Control extracts were also analyzed. Immunoprecipitations were performed in the absence (lanes 1–8), and presence (lanes 9–20) of p50 antibody. The immunoprecipitated portion (IP) and 25% of the supernatant (S) were analyzed by Western blot using Flag and p50 antibodies. Extracts containing fp65 were adjusted by dilution so that comparable concentrations of fp65 were present in each reaction. Extracts containing fc-Rel were also adjusted. It is noteworthy the p50 antibody detected and precipitated the 293T-cell endogenous human p50 protein (e.g., lane 2), but did not coprecipitate detectable amounts of fc-Rel and fp65 in the absence of overexpressed murine p50. A likely explanation is that the endogenous human p50 was in fact present at a much lower concentration than the overexpressed murine p50, but yielded a strong signal on the Western blots because the p50 antibody was raised against the human protein and presumably bound the human protein with a higher affinity. (C) Gel shifts were performed with extracts from 293T cells (4 μg) containing p50/fc-Rel (lanes 1–7) or p50/fp65 (lanes 8–14) complexes overexpressed to similar levels. Probes containing the wild-type p40 Rel site (lanes 7 and 14) and various 3-bp mutations (lanes 1–6, 8–13) were analyzed. The effect of each mutation on promoter activity in a transient transfection assay is indicated at the bottom (6). Also indicated is the ratio of p50 homodimer to p50/fp65 or p50/fc-Rel heterodimer.
Figure 3
Comparable transactivation of a p40 promoter-reporter plasmid by p50/p65 and p50/c-Rel. 293T cells were transfected with 2 μg of a wild-type p40 promoter-luciferase reporter plasmid (ref. ; lanes 1–6 and 11–14) or a plasmid containing a Rel-site mutation (lanes 7–10 and 15–18). Reporter plasmids were transfected alone (lanes 1, 7, and 15) or with a p50 expression plasmid (0.1 μg; lane 2) and increasing amounts of fc-Rel (2–16 μg; lanes 3–6 and 8–10) or fp65 (0.2–1.6 μg; lanes 11–14 and 16–18) expression plasmids. The fold-induction values were determined by dividing the luciferase activity by the basal activity of the reporter alone (lane 1 for the wild-type and lanes 8 and 16 for the mutant constructs). fc-Rel, fp65, and p50 were monitored by Western blot (Lower). The p50 detected in lanes 1, 7, and 15 corresponds to endogenous human p50.
Figure 4
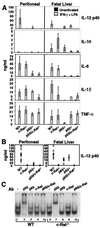
A selective defect in IL-12 p40 production in c-Rel−/− macrophages. (A) Cytokine expression by peritoneal (Left) or fetal-liver (Right) macrophages was monitored by ELISA. Error bars represent the range of results rather than the standard deviation. (B) The p40 ELISA data from each sample analyzed during this study are shown (○). The ● indicate the average. (C) Gel shift assays were performed with extracts from wild-type (lanes 1–5) and c-Rel−/− (lanes 6–10) peritoneal macrophages activated with LPS/IFN-γ for 4 h.
Figure 5
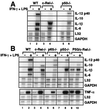
Steady-state mRNA levels in wild-type and Rel-deficient macrophages. Total RNA was isolated from peritoneal (A) and fetal liver-derived (B) macrophages before and 4 h after activation with LPS/IFN-γ. Specific mRNA levels were determined by RNase protection. The data shown correspond only to the cytokine mRNAs analyzed by ELISA in Fig. 4.
Similar articles
- CpG ODN-mediated regulation of IL-12 p40 transcription.
Takeshita F, Klinman DM. Takeshita F, et al. Eur J Immunol. 2000 Jul;30(7):1967-76. doi: 10.1002/1521-4141(200007)30:7<1967::AID-IMMU1967>3.0.CO;2-5. Eur J Immunol. 2000. PMID: 10940886 - A proximal kappaB site in the IL-23 p19 promoter is responsible for RelA- and c-Rel-dependent transcription.
Mise-Omata S, Kuroda E, Niikura J, Yamashita U, Obata Y, Doi TS. Mise-Omata S, et al. J Immunol. 2007 Nov 15;179(10):6596-603. doi: 10.4049/jimmunol.179.10.6596. J Immunol. 2007. PMID: 17982049
Cited by
- The noncanonical NFκB pathway: Regulatory mechanisms in health and disease.
Rodriguez BN, Huang H, Chia JJ, Hoffmann A. Rodriguez BN, et al. WIREs Mech Dis. 2024 Nov-Dec;16(6):e1646. doi: 10.1002/wsbm.1646. Epub 2024 Apr 18. WIREs Mech Dis. 2024. PMID: 38634218 Review. - Co-imaging of RelA and c-Rel reveals features of NF-κB signaling for ligand discrimination.
Rahman SMT, Singh A, Lowe S, Aqdas M, Jiang K, Vaidehi Narayanan H, Hoffmann A, Sung MH. Rahman SMT, et al. Cell Rep. 2024 Mar 26;43(3):113940. doi: 10.1016/j.celrep.2024.113940. Epub 2024 Mar 13. Cell Rep. 2024. PMID: 38483906 Free PMC article. - IL-10 constrains sphingolipid metabolism to limit inflammation.
York AG, Skadow MH, Oh J, Qu R, Zhou QD, Hsieh WY, Mowel WK, Brewer JR, Kaffe E, Williams KJ, Kluger Y, Smale ST, Crawford JM, Bensinger SJ, Flavell RA. York AG, et al. Nature. 2024 Mar;627(8004):628-635. doi: 10.1038/s41586-024-07098-5. Epub 2024 Feb 21. Nature. 2024. PMID: 38383790 Free PMC article. - The IRAK1/IRF5 axis initiates IL-12 response by dendritic cells and control of Toxoplasma gondii infection.
Pereira M, Ramalho T, Andrade WA, Durso DF, Souza MC, Fitzgerald KA, Golenbock DT, Silverman N, Gazzinelli RT. Pereira M, et al. Cell Rep. 2024 Feb 27;43(2):113795. doi: 10.1016/j.celrep.2024.113795. Epub 2024 Feb 15. Cell Rep. 2024. PMID: 38367238 Free PMC article. - Exogenous IL-25 ameliorates airway neutrophilia via suppressing macrophage M1 polarization and the expression of IL-12 and IL-23 in asthma.
Chang C, Chen G, Wu W, Chen D, Chen S, Gao J, Feng Y, Zhen G. Chang C, et al. Respir Res. 2023 Oct 28;24(1):260. doi: 10.1186/s12931-023-02557-5. Respir Res. 2023. PMID: 37898756 Free PMC article.
References
- Sweet M J, Hume D A. J Leukocyte Biol. 1996;60:8–26. - PubMed
- Gately M K, Renzetti L M, Magram J, Stern A S, Adorini L, Gubler U, Presky D H. Annu Rev Immunol. 1998;16:495–521. - PubMed
- Trinchieri G. Adv Immunol. 1998;70:83–243. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials