Cytokeratins 8 and 19 in the mouse placental development - PubMed (original) (raw)
Cytokeratins 8 and 19 in the mouse placental development
Y Tamai et al. J Cell Biol. 2000.
Abstract
To investigate the expression and biological roles of cytokeratin 19 (K19) in development and in adult tissues, we inactivated the mouse K19 gene (Krt1-19) by inserting a bacterial beta-galactosidase gene (lacZ) by homologous recombination in embryonic stem cells, and established germ line mutant mice. Both heterozygous and homozygous mutant mice were viable, fertile, and appeared normal. By 7.5-8.0 days post coitum (dpc), heterozygous mutant embryos expressed lacZ in the notochordal plate and hindgut diverticulum, reflecting the fact that the notochord and the gut endoderm are derived from the axial mesoderm-originated cells. In the adult mutant, lacZ was expressed mainly in epithelial tissues. To investigate the possible functional cooperation and synergy between K19 and K8, we then constructed compound homozygous mutants, whose embryos died approximately 10 dpc. The lethality resulted from defects in the placenta where both K19 and K8 are normally expressed. As early as 9. 5 dpc, the compound mutant placenta had an excessive number of giant trophoblasts, but lacked proper labyrinthine trophoblast or spongiotrophoblast development, which apparently caused flooding of the maternal blood into the embryonic placenta. These results indicate that K19 and K8 cooperate in ensuring the normal development of placental tissues.
Figures
Figure 1
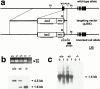
Inactivation of the mouse K19 gene (Krt1-19) by homologous recombination. (a) Structures of the wild-type allele, targeting vector pJBX, and the targeted allele. The targeting vector contained two regions of homology to the genomic DNA, sandwiching a lacZ reporter-neo selection cassette. Homologous recombination resulted in deletion of most of exon 1, and the lacZ reporter was transcribed from the Krt1-19 promoter. Filled boxes represent exons; noncoding regions are shown as solid lines. Arrowheads indicate the positions of the PCR primers for genotyping: KO PCR, primers for the targeted allele, and WT PCR, primers for the wild-type allele. The probe for Southern hybridization is shown as a solid line. RI, EcoRI site. Arrows beneath the lacZ reporter-neo selection cassette show the transcriptional directions. (b) Genotype analysis of the wild-type, heterozygous, and homozygous mutant mouse tail DNAs. (Top) Transmission of the targeted allele to the progeny as determined by PCR. KO, targeted allele (739 bp); WT, wild-type allele (958 bp). (Bottom) Southern hybridization analysis. The EcoRI fragments of 4.5 and 1.6 kb derived from the wild-type and targeted alleles, respectively. (c) Northern hybridization analysis of K19 mRNA in the wild-type, heterozygous, and homozygous mice. 10 μg of the total RNA purified from the colon (c), or small intestine (i) were loaded in each lane and probed with a K19 cDNA probe (Ichinose et al. 1989). The arrowhead indicates the size of the K19 mRNA (1.4 kb).
Figure 2
Immunofluorescence staining of K19, K8, K18, and K7 in the K19 gene heterozygous and homozygous mutant mouse intestines. Frozen sections of the adult mice were fixed with methanol and stained with TROMA-3 (anti–K19), -1 (anti–K8), and -2 (anti–K18), and an anti–K7 mAb.
Figure 3
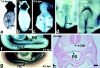
Expression of the β-galactosidase gene (Krt1-19-lacZ) in the heterozygous mouse embryos at 7.5–9.0 dpc. (a and b) A representative embryo at 7.5 dpc in the anterior and posterior views, respectively. Note the β-galactosidase activity in the notochordal plate, node, and ectoplacental cone. (c) A representative embryo at 8.5 dpc in the posterior view. Note the β-galactosidase activity in an arch pattern (arrows). (d and e) Another embryo at 8.5 dpc in the anterior (d) and posterior (e) views. Note that this embryo is more progressed in development than that in c: the arch pattern of the β-galactosidase staining at the hindgut (e, arrows) is much smaller than that seen in the embryo shown in c. Also note the staining inside the foregut in d (arrow). (f and g) A representative embryo at 9.0 dpc. (h) A transverse section of the same embryo shown in f and g (Eosin counterstaining). hf, head fold; nc, notochordal plate; nd, node; pg, primitive gut. All dissection micrographs (whole mount in situ staining) were taken under a dark-field illumination, except a and g, which were in a bright field. Bar, 100 μm.
Figure 4
Colocalization of K8 and K19 in the mouse extra embryonic tissues. Immunohistochemical staining of the wild-type embryos in utero at 7.5 dpc with TROMA-3 (anti–K19), TROMA-1 (anti–K8), and anti–Kip2 antibodies. Note that K19 and K8 are expressed in the cytoplasm, whereas Kip2 is in the nucleus. (a and b) Staining for K19. (c and d) Staining for K8. (e and f) Staining for Kip2. epc, ectoplacental cone; gc, giant cells; glc, glycogen cells; pe, parietal yolk sac endoderm; ve, visceral yolk sac endoderm. Note that a, c, and e are serial sections, while b, d, and f are a higher magnification of the sections in a, c, and e, respectively, Bars: a, c, and e, 100 μm; b, d, and f, 50 μm.
Figure 7
Degeneration of the placenta in the K8/19 compound homozygous concepti at 9.5 dpc; comparison with the K19 homozygous controls. (a–f) Double staining with X-gal for the β-galactosidase activity (greenish blue), and with a specific antibody for Kip2 (brown), followed by a light counterstaining with hematoxylin. Note that the greenish tinge of the blue color for X-gal is due to an electronic enhancement; and that Kip2 is expressed in the nuclei. *The placental lesion; al, allantois; gc, giant cells; lb, labyrinthine trophoblast; and sp, spongiotrophoblast. (g and h) Staining for K7 by indirect immunofluorescence microscopy. Note that image g was electronically enhanced more than h. (Left) Samples of the K8/K19 compound homozygotes [Krt2-8 (−/−):Krt1-19 (−/−)]. (Right) Samples of the simple K19 homozygotes [Krt2-8 (+/+):Krt1-19 (−/−)]. Bars: (a–f) 100 μm; (g and h) 1 μm.
Figure 5
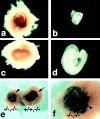
Smaller placentas and embryo growth retardation in the K8/K19 compound homozygous mutant concepti. (a) A Krt2-8 (−/−):Krt1-19 (−/−) conceptus at 9.75 dpc. Note a red lesion (arrowhead). (b) A Krt2-8 (−/−):Krt1-19 (−/−) embryo, showing a retarded growth. (c) A Krt2-8 (+/+):Krt1-19 (−/−) conceptus at 9.75 dpc. Arrow indicates the implantation site. (d) A Krt2-8 (+/+):Krt1-19 (−/−) embryo as a control. Note that a and c, and b and d, were photographed at the same magnifications. (e) Comparison of the placenta between a K8/K19 compound homozygote and a simple K19 homozygote, stained for β-galactosidase at 9.75 dpc. (−/−,−/−) Compound homozygote [i.e., Krt2-8 (−/−):Krt1-19 (−/−)]; (+/+,−/−) simple K19 homozygote [Krt2-8 (+/+):Krt1-19 (−/−)]. (f) A higher magnification of the compound homozygous conceptus in e.
Figure 6
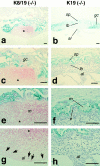
Comparison of the placental regions at 10.5 dpc between the K8/K19 compound homozygotes and simple K19 homozygotes. (Left) Sections from the K8/K19 compound homozygous concepti [Krt2-8 (−/−):Krt1-19 (−/−)]. (Right) Sections from the simple K19 homozygous concepti [Krt2-8 (+/+):Krt1-19 (−/−)]. (Stained for β-galactosidase with hematoxylin counterstaining.) Note the lesion with the maternal blood (*) and the degenerated allantoic tissue with less vascularization compared with the healthy giant cells and the spongiotrophoblasts in b, d, f and h. In the normal placenta, β-galactosidase (K19) was expressed in the giant cells and the spongiotrophoblasts, but not in the allantois or the labyrinthine trophoblasts. Note the degeneration in the spongiotrophoblasts and the labyrinthine trophoblasts in the compound homozygotes. Arrows in g indicate nucleated embryonic erythrocytes. al, allantois; e, embryonic blood; gc, giant cells; lb, labyrinthine trophoblast; m, maternal blood; sp, spongiotrophoblast. Bars, 100 μm.
Similar articles
- Targeted deletion of keratins 18 and 19 leads to trophoblast fragility and early embryonic lethality.
Hesse M, Franz T, Tamai Y, Taketo MM, Magin TM. Hesse M, et al. EMBO J. 2000 Oct 2;19(19):5060-70. doi: 10.1093/emboj/19.19.5060. EMBO J. 2000. PMID: 11013209 Free PMC article. - Keratin 8 protection of placental barrier function.
Jaquemar D, Kupriyanov S, Wankell M, Avis J, Benirschke K, Baribault H, Oshima RG. Jaquemar D, et al. J Cell Biol. 2003 May 26;161(4):749-56. doi: 10.1083/jcb.200210004. J Cell Biol. 2003. PMID: 12771125 Free PMC article. - Keratin 5-Cre-driven excision of nonmuscle myosin IIA in early embryo trophectoderm leads to placenta defects and embryonic lethality.
Crish J, Conti MA, Sakai T, Adelstein RS, Egelhoff TT. Crish J, et al. Dev Biol. 2013 Oct 1;382(1):136-48. doi: 10.1016/j.ydbio.2013.07.017. Epub 2013 Jul 30. Dev Biol. 2013. PMID: 23911870 Free PMC article. - Mst1 and mst2 are essential regulators of trophoblast differentiation and placenta morphogenesis.
Du X, Dong Y, Shi H, Li J, Kong S, Shi D, Sun LV, Xu T, Deng K, Tao W. Du X, et al. PLoS One. 2014 Mar 4;9(3):e90701. doi: 10.1371/journal.pone.0090701. eCollection 2014. PLoS One. 2014. PMID: 24595170 Free PMC article. - Skp2 contributes to cell cycle progression in trophoblast stem cells and to placental development.
Yamauchi Y, Nita A, Nishiyama M, Muto Y, Shimizu H, Nakatsumi H, Nakayama KI. Yamauchi Y, et al. Genes Cells. 2020 Jun;25(6):427-438. doi: 10.1111/gtc.12769. Epub 2020 Apr 22. Genes Cells. 2020. PMID: 32267063
Cited by
- High Keratin 8/18 Ratio Predicts Aggressive Hepatocellular Cancer Phenotype.
Golob-Schwarzl N, Bettermann K, Mehta AK, Kessler SM, Unterluggauer J, Krassnig S, Kojima K, Chen X, Hoshida Y, Bardeesy NM, Müller H, Svendova V, Schimek MG, Diwoky C, Lipfert A, Mahajan V, Stumptner C, Thüringer A, Fröhlich LF, Stojakovic T, Nilsson KPR, Kolbe T, Rülicke T, Magin TM, Strnad P, Kiemer AK, Moriggl R, Haybaeck J. Golob-Schwarzl N, et al. Transl Oncol. 2019 Feb;12(2):256-268. doi: 10.1016/j.tranon.2018.10.010. Epub 2018 Nov 12. Transl Oncol. 2019. PMID: 30439626 Free PMC article. - Self-Organization of Mouse Stem Cells into an Extended Potential Blastoid.
Sozen B, Cox AL, De Jonghe J, Bao M, Hollfelder F, Glover DM, Zernicka-Goetz M. Sozen B, et al. Dev Cell. 2019 Dec 16;51(6):698-712.e8. doi: 10.1016/j.devcel.2019.11.014. Dev Cell. 2019. PMID: 31846649 Free PMC article. - γδ T cells compose a developmentally regulated intrauterine population and protect against vaginal candidiasis.
Monin L, Ushakov DS, Arnesen H, Bah N, Jandke A, Muñoz-Ruiz M, Carvalho J, Joseph S, Almeida BC, Green MJ, Nye E, Hatano S, Yoshikai Y, Curtis M, Carlsen H, Steinhoff U, Boysen P, Hayday A. Monin L, et al. Mucosal Immunol. 2020 Nov;13(6):969-981. doi: 10.1038/s41385-020-0305-7. Epub 2020 May 29. Mucosal Immunol. 2020. PMID: 32472066 Free PMC article. - Reg-II is an exocrine pancreas injury-response product that is up-regulated by keratin absence or mutation.
Zhong B, Strnad P, Toivola DM, Tao GZ, Ji X, Greenberg HB, Omary MB. Zhong B, et al. Mol Biol Cell. 2007 Dec;18(12):4969-78. doi: 10.1091/mbc.e07-02-0180. Epub 2007 Sep 26. Mol Biol Cell. 2007. PMID: 17898082 Free PMC article. - Effects of peroxisome proliferator-activated receptor delta on placentation, adiposity, and colorectal cancer.
Barak Y, Liao D, He W, Ong ES, Nelson MC, Olefsky JM, Boland R, Evans RM. Barak Y, et al. Proc Natl Acad Sci U S A. 2002 Jan 8;99(1):303-8. doi: 10.1073/pnas.012610299. Epub 2001 Dec 26. Proc Natl Acad Sci U S A. 2002. PMID: 11756685 Free PMC article.
References
- Bader B.L., Jahn L., Franke W.W. Low level expression of cytokeratin 8, 18 and 19 in vascular smooth muscle cells of human umbilical cord and in cultured cells derived therefrom, with an analysis of the chromosomal locus containing the cytokeratin 19 gene. Eur. J. Cell Biol. 1988;47:300–319. - PubMed
- Baribault H., Price J., Miyai K., Oshima R.G. Mid-gestational lethality in mice lacking keratin 8. Genes Dev. 1993;7:1191–1202. - PubMed
- Baribault H., Penner J., Iozzo R.V., Wilson-Heiner M. Colorectal hyperplasia and inflammation in keratin 8-deficient FVB/N mice. Genes Dev. 1994;8:2964–2973. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials