Differential gene expression in p53-mediated apoptosis-resistant vs. apoptosis-sensitive tumor cell lines - PubMed (original) (raw)
Differential gene expression in p53-mediated apoptosis-resistant vs. apoptosis-sensitive tumor cell lines
S A Maxwell et al. Proc Natl Acad Sci U S A. 2000.
Abstract
Induction of wild-type p53 in the ECV-304 bladder carcinoma cell line by infection with a p53 recombinant adenovirus (Ad5CMV-p53) resulted in extensive apoptosis and eventual death of nearly all of the cells. As a strategy to determine the molecular events important to p53-mediated apoptosis in these transformed cells, ECV-304 cells were selected for resistance to p53 by repeated infections with Ad5CMV-p53. We compared the expression of 5,730 genes in p53-resistant (DECV) and p53-sensitive ECV-304 cells by reverse transcription-PCR, Northern blotting, and DNA microarray analysis. The expression of 480 genes differed by 2-fold or more between the two p53-infected cell lines. A number of potential targets for p53 were identified that play roles in cell cycle regulation, DNA repair, redox control, cell adhesion, apoptosis, and differentiation. Proline oxidase, a mitochondrial enzyme involved in the proline/pyrroline-5-carboxylate redox cycle, was up-regulated by p53 in ECV but not in DECV cells. Pyrroline-5-carboxylate (P5C), a proline-derived metabolite generated by proline oxidase, inhibited the proliferation and survival of ECV-304 and DECV cells and induced apoptosis in both cell lines. A recombinant proline oxidase protein tagged with a green fluorescent protein at the amino terminus localized to mitochondria and induced apoptosis in p53-null H1299 non-small cell lung carcinoma cells. The results directly implicate proline oxidase and the proline/P5C pathway in p53-induced growth suppression and apoptosis.
Figures
Figure 1
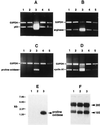
Differential regulation of gene expression by p53 in ECV-304 and DECV cells. ECV-304 and DECV cells were infected with control recombinant β-galactosidase adenovirus (ECV/gal; DECV/gal) or recombinant p53 adenovirus (ECV/p53; DECV/p53) for 12 h. Total RNA was extracted and subjected to RT-PCR to analyze the expression of_XRCC9, Bcl-2, PISSLRE, BTG2, Apaf-1, Bax, Pig-3, Pig-11, Bcl_ XL , E2F, Bak, protocadherin-42 (PRCAD-42), cyclin A1 (CYCLIN A), and HLH 1R21 (HLHP). Glyceraldehyde 3-phosphate dehydrogenase and Bad, which were not influenced by p53, were used as expression controls to ensure that equivalent amounts of cDNA were included in each assay.
Figure 2
Induction of proline oxidase and arginase II in ECV-304 cells up-regulated for p53. (A–D) RT-PCR was performed to analyze p53 (A), arginase II (B), proline oxidase (C), and cyclin A1 (D) in mock-infected (lane 1), GFP recombinant adenovirus-infected (lane 2), p53 recombinant adenovirus-infected (lane 3), DMSO-treated (lane 4), and P5C-treated (lane 5) ECV-304 cells. (E) Northern blot analysis was performed on total RNA isolated from mock-infected (lane 1), GFP-infected (lane 2), and p53-infected (lane 3) ECV-304 cells using a radiolabeled proline oxidase cDNA probe and shows induction of proline oxidase mRNA only in p53-induced cells. (F) After capillary transfer from the agarose gel to a nylon membrane, total RNA was stained with methylene blue before hybridization with the proline oxidase probe and shows equivalent amounts of quality total RNA in each gel lane.
Figure 3
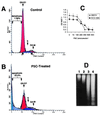
P5C inhibits growth and induces apoptosis in p53-sensitive and p53-resistant cells. (A and B) FACS was performed on ECV-304 cells treated with DMSO (A) and on cells treated with P5C (B) for 16 h. (C) P5C inhibited the proliferation and reduced the survival of DECV and ECV-304 cells. (D) Internucleosomal DNA fragmentation analysis supported the FACS analysis in showing DNA fragmentation ladders characteristic of apoptosis. DMSO-treated ECV-304 and DECV are shown in lanes 1 and 2, and ECV-304 and DECV cells treated with 600 μM P5C for 36 h are shown in lanes 3 and 4, respectively.
Figure 4
A recombinant GFP-proline oxidase induces apoptosis in H1299 non-small cell lung carcinoma cells. (A) H1299 cells were transfected with GFP (lane 1) or GFP-proline oxidase (lane 2) expression vector for 24 h and immunoblotted with a polyclonal GFP antibody. (B) H1299 cells at 40–50% confluence were transfected with GFP or GFP-proline oxidase and subjected to FACS analyses 72 h later. (C) Quantitation of apoptosis occurring in cells transfected with GFP, GFP-proline oxidase fusion protein, or a combination of GFP + p53. The data values shown were compiled from four independent FACS scans.
Figure 5
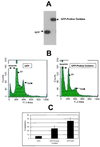
Recombinant GFP-proline oxidase localizes to mitochondria. Cells were subfractionated into nuclei (N), cytoplasmic/membrane (C), and mitochondrial (M) fractions. (A and B) A portion of each subcellular fraction (5 μg protein) was solubilized in SDS gel loading buffer and immunoblotted with a polyclonal GFP antibody (A) or a monoclonal cytochrome c antibody (B). (C) Confocal microscopy was used to visualize the localization of GFP-proline oxidase in transfected H1299 cells (GFP-POX; green fluorescence). These same cells were counterstained with MitoTracker Red (red fluorescence) to visualize the localized concentration of mitochondria in the cytoplasm. The right panel shows the simultaneous excitation of GFP-proline oxidase and MitoTracker Red in the same field as that shown in the left and middle panels, which yielded a yellowish-red fluorescence at areas of colocalization of GFP-POX and MitoTracker Red. Filtered control analyses indicated that there was no contamination of the red fluorescence window by the green fluorescence signal or of the green fluorescence window by the red fluorescence signal.
Similar articles
- Biological and molecular characterization of an ECV-304-derived cell line resistant to p53-mediated apoptosis.
Maxwell SA, Davis GE. Maxwell SA, et al. Apoptosis. 2000 Jun;5(3):277-90. doi: 10.1023/a:1009660714216. Apoptosis. 2000. PMID: 11225849 - Gene expression profiling of p53-sensitive and -resistant tumor cells using DNA microarray.
Maxwell SA, Davis GE. Maxwell SA, et al. Apoptosis. 2004 Mar;9(2):171-9. doi: 10.1023/B:APPT.0000018799.20120.38. Apoptosis. 2004. PMID: 15004514 - Enhancement of radiosensitivity of wild-type p53 human glioma cells by adenovirus-mediated delivery of the p53 gene.
Lang FF, Yung WK, Raju U, Libunao F, Terry NH, Tofilon PJ. Lang FF, et al. J Neurosurg. 1998 Jul;89(1):125-32. doi: 10.3171/jns.1998.89.1.0125. J Neurosurg. 1998. PMID: 9647183 - Proline dehydrogenase (oxidase) in cancer.
Liu W, Phang JM. Liu W, et al. Biofactors. 2012 Nov-Dec;38(6):398-406. doi: 10.1002/biof.1036. Epub 2012 Aug 8. Biofactors. 2012. PMID: 22886911 Free PMC article. Review. - Proline metabolism and microenvironmental stress.
Phang JM, Liu W, Zabirnyk O. Phang JM, et al. Annu Rev Nutr. 2010 Aug 21;30:441-63. doi: 10.1146/annurev.nutr.012809.104638. Annu Rev Nutr. 2010. PMID: 20415579 Free PMC article. Review.
Cited by
- Tumor-derived p53 mutants induce NF-kappaB2 gene expression.
Scian MJ, Stagliano KE, Anderson MA, Hassan S, Bowman M, Miles MF, Deb SP, Deb S. Scian MJ, et al. Mol Cell Biol. 2005 Nov;25(22):10097-110. doi: 10.1128/MCB.25.22.10097-10110.2005. Mol Cell Biol. 2005. PMID: 16260623 Free PMC article. - A Molecular Dynamics (MD) and Quantum Mechanics/Molecular Mechanics (QM/MM) study on Ornithine Cyclodeaminase (OCD): a tale of two iminiums.
Ion BF, Bushnell EA, Luna PD, Gauld JW. Ion BF, et al. Int J Mol Sci. 2012 Oct 11;13(10):12994-3011. doi: 10.3390/ijms131012994. Int J Mol Sci. 2012. PMID: 23202934 Free PMC article. - Phospho-ΔNp63α/SREBF1 protein interactions: bridging cell metabolism and cisplatin chemoresistance.
Huang Y, Bell LN, Okamura J, Kim MS, Mohney RP, Guerrero-Preston R, Ratovitski EA. Huang Y, et al. Cell Cycle. 2012 Oct 15;11(20):3810-27. doi: 10.4161/cc.22022. Epub 2012 Sep 5. Cell Cycle. 2012. PMID: 22951905 Free PMC article. - Proline modulates the intracellular redox environment and protects mammalian cells against oxidative stress.
Krishnan N, Dickman MB, Becker DF. Krishnan N, et al. Free Radic Biol Med. 2008 Feb 15;44(4):671-81. doi: 10.1016/j.freeradbiomed.2007.10.054. Epub 2007 Nov 12. Free Radic Biol Med. 2008. PMID: 18036351 Free PMC article. - Functional consequences of PRODH missense mutations.
Bender HU, Almashanu S, Steel G, Hu CA, Lin WW, Willis A, Pulver A, Valle D. Bender HU, et al. Am J Hum Genet. 2005 Mar;76(3):409-20. doi: 10.1086/428142. Epub 2005 Jan 20. Am J Hum Genet. 2005. PMID: 15662599 Free PMC article.
References
- Martinez J, Georgoff I, Martinez J, Levine A J. Genes Dev. 1991;5:151–159. - PubMed
- Alonigrinstein R, Zanbar I, Alboum I, Goldfinger N, Rotter V. Oncogene. 1993;8:3297–3305. - PubMed
- Yin Y, Tainsky M A, Bischoff F Z, Strong L C, Wahl G M. Cell. 1992;70:937–948. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous