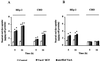High cell sensitivity to Helicobacter pylori VacA toxin depends on a GPI-anchored protein and is not blocked by inhibition of the clathrin-mediated pathway of endocytosis - PubMed (original) (raw)
High cell sensitivity to Helicobacter pylori VacA toxin depends on a GPI-anchored protein and is not blocked by inhibition of the clathrin-mediated pathway of endocytosis
V Ricci et al. Mol Biol Cell. 2000 Nov.
Free PMC article
Abstract
Helicobacter pylori vacuolating toxin (VacA) causes vacuolation in a variety of cultured cell lines, sensitivity to VacA differing greatly, however, among the different cell types. We found that the high sensitivity of HEp-2 cells to VacA was impaired by treating the cells with phosphatidylinositol-specific phospholipase C (PI-PLC) which removes glycosylphosphatidylinositol (GPI)-anchored proteins from the cell surface. Incubation of cells with a cholesterol-sequestering agent, that impairs both structure and function of sphingolipid-cholesterol-rich membrane microdomains ("lipid rafts"), also impaired VacA-induced cell vacuolation. Overexpression into HEp-2 cells of proteins inhibiting clathrin-dependent endocytosis (i.e., a dominant-negative mutant of Eps15, the five tandem Src-homology-3 domains of intersectin, and the K44A dominant-negative mutant of dynamin II) did not affect vacuolation induced by VacA. Nevertheless, F-actin depolymerization, known to block the different types of endocytic mechanisms, strongly impaired VacA vacuolating activity. Taken together, our data suggest that the high cell sensitivity to VacA depends on the presence of one or several GPI-anchored protein(s), intact membrane lipid rafts, and an uptake mechanism via a clathrin-independent endocytic pathway.
Figures
Figure 1
Neutral red uptake by HEp-2 and CHO cells variously exposed to VacA. (A) Cells were incubated for either 5 h or 16 h at 37°C with VacA+ BCF or purified VacA in HBSS containing 5 mM NH4Cl (see Methods). (B) Cells were exposed to VacA+ BCF or purified VacA in HBSS for 1 h at 4°C and then, after extensive washing, incubated for either 5 h or 16 h in HBSS containing 5 mM NH4Cl (see Methods). Controls were paired monolayers not exposed to VacA. Means ± SEM of three independent experiments. * = p < 0.05 versus paired Control. ° = p < 0.05 versus the same condition at 5 h.
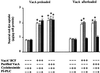
Figure 2
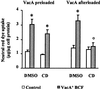
Effect of PI-PLC treatment on VacA-induced vacuolation of HEp-2 cells. Neutral red uptake was measured either 1) in cells previously exposed to VacA pulse (both as VacA+ BCF and as purified VacA) and then treated or not with PI-PLC (VacA preloaded), or 2) in cells treated or not with PI-PLC and then exposed or not to VacA pulse (VacA afterloaded) (see Methods). Incubation for vacuole development: 3 h at 37°C in HBSS containing 5 mM NH4Cl. Means ± SEM of three independent experiments.* = p < 0.05 versus paired monolayers not exposed to VacA.
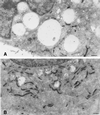
Figure 3
Ultrastuctural features of HEp-2 cells treated or not with PI-PLC and then exposed to VacA. Cells untreated (A) or treated with PI-PLC (B) were incubated for 10 min with VacA+ BCF and then for 3 h with HBSS containing 5 mM NH4Cl (see Methods). Note large vacuoles filled with intense VacA immunoreactivity in A, to be compared with the small vacuoles and scarce VacA immunoreactivity in B. Aldehyde-osmium fixation, VacA immunogold, uranyl-lead counterstaining. Bars = 0.85 μm.
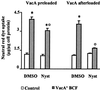
Figure 4
Effect of nystatin (Nyst) treatment on VacA-induced vacuolation of HEp-2 cells. Neutral red uptake was measured either 1) in cells previously exposed to VacA+ BCF pulse, or in control cells and then treated with Nyst dissolved in DMSO or with DMSO alone (VacA preloaded); or 2) in cells treated with Nyst dissolved in DMSO, or with DMSO alone and then exposed or not (Control) to VacA+ BCF pulse (VacA afterloaded) (see Methods). Incubation for vacuole development: 5 h at 37°C in HBSS containing 5 mM NH4Cl. Means = SEM of three independent experiments.* = p < 0.05 versus paired Control. ° = p < 0.05 versus the same condition but DMSO-treated.
Figure 5
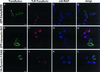
VacA-induced vacuolation in HEp-2 cells overexpressing proteins specifically inhibiting the formation of clathrin-coated vesicles. HEp-2 cells grown on coverslip were transiently transfected with vectors allowing overexpression of 1) a dominant-negative mutant of the Eps15 protein, tagged with GFP (GFP-Edelta95/295) (A-D); 2) the five tandem SH3 domains of intersectin, tagged with GFP (GFP-Intersectin-SH3) (E-H); and 3) the K44A dominant-negative mutant of dynamin II, tagged with a HA epitope (HA-Dynamin II K44A) (I-L). Transfected monolayers were exposed to VacA+ BCF pulse, incubated for 16 h at 37°C in HBSS containing 3 mM NH4Cl, and then tested for TxR-Transferrin endocytosis as described in Methods. After fixations, cells were processed for immunofluorescence to detect Rab7-positive vacuoles and, where required, HA tag. (A, E, I): transfected cells (green). (B, F, J): TxR-Transferrin (red). (C, G, K): Rab7 localization (cyan). (D, H, L): merge images showing several Rab7-positive vacuoles in transfected cells where there was virtually no uptake of TxR-Transferrin. Bars = 10 μm.
Figure 6
Effect of cytochalasin D (CD) treatment on VacA-induced vacuolation of HEp-2 cells. Neutral red uptake was measured either 1) in cells previously exposed to VacA+ BCF pulse, or in control cells and then treated with CD dissolved in DMSO or with DMSO alone (VacA preloaded); or 2) in cells treated with CD dissolved in DMSO, or with DMSO alone and then exposed or not (Control) to VacA+ BCF pulse (VacA afterloaded) (see Methods). Incubation for vacuole development: 5 h at 37°C in HBSS containing 5 mM NH4Cl. Means = SEM of three independent experiments. * = p < 0.05 versus paired Control. ° = p < 0.05 versus the same condition but DMSO-treated.
Similar articles
- Association of Helicobacter pylori vacuolating toxin (VacA) with lipid rafts.
Schraw W, Li Y, McClain MS, van der Goot FG, Cover TL. Schraw W, et al. J Biol Chem. 2002 Sep 13;277(37):34642-50. doi: 10.1074/jbc.M203466200. Epub 2002 Jul 16. J Biol Chem. 2002. PMID: 12121984 - Glycosylphosphatidylinositol-anchored proteins and actin cytoskeleton modulate chloride transport by channels formed by the Helicobacter pylori vacuolating cytotoxin VacA in HeLa cells.
Gauthier NC, Ricci V, Gounon P, Doye A, Tauc M, Poujeol P, Boquet P. Gauthier NC, et al. J Biol Chem. 2004 Mar 5;279(10):9481-9. doi: 10.1074/jbc.M312040200. Epub 2003 Dec 14. J Biol Chem. 2004. PMID: 14676190 - Helicobacter pylori VacA cytotoxin: a probe for a clathrin-independent and Cdc42-dependent pinocytic pathway routed to late endosomes.
Gauthier NC, Monzo P, Kaddai V, Doye A, Ricci V, Boquet P. Gauthier NC, et al. Mol Biol Cell. 2005 Oct;16(10):4852-66. doi: 10.1091/mbc.e05-05-0398. Epub 2005 Jul 29. Mol Biol Cell. 2005. PMID: 16055501 Free PMC article. - Vacuolating cytotoxin A (VacA) - A multi-talented pore-forming toxin from Helicobacter pylori.
Junaid M, Linn AK, Javadi MB, Al-Gubare S, Ali N, Katzenmeier G. Junaid M, et al. Toxicon. 2016 Aug;118:27-35. doi: 10.1016/j.toxicon.2016.04.037. Epub 2016 Apr 20. Toxicon. 2016. PMID: 27105670 Review. - Helicobacter pylori VacA, a distinct toxin exerts diverse functionalities in numerous cells: An overview.
Chauhan N, Tay ACY, Marshall BJ, Jain U. Chauhan N, et al. Helicobacter. 2019 Feb;24(1):e12544. doi: 10.1111/hel.12544. Epub 2018 Oct 16. Helicobacter. 2019. PMID: 30324717 Review.
Cited by
- Outer Membrane Vesicle Production by Helicobacter pylori Represents an Approach for the Delivery of Virulence Factors CagA, VacA and UreA into Human Gastric Adenocarcinoma (AGS) Cells.
Chew Y, Chung HY, Lin PY, Wu DC, Huang SK, Kao MC. Chew Y, et al. Int J Mol Sci. 2021 Apr 11;22(8):3942. doi: 10.3390/ijms22083942. Int J Mol Sci. 2021. PMID: 33920443 Free PMC article. - Relationship between VacA Toxin and Host Cell Autophagy in Helicobacter pylori Infection of the Human Stomach: A Few Answers, Many Questions.
Ricci V. Ricci V. Toxins (Basel). 2016 Jul 1;8(7):203. doi: 10.3390/toxins8070203. Toxins (Basel). 2016. PMID: 27376331 Free PMC article. Review. - Clathrin-independent carriers form a high capacity endocytic sorting system at the leading edge of migrating cells.
Howes MT, Kirkham M, Riches J, Cortese K, Walser PJ, Simpson F, Hill MM, Jones A, Lundmark R, Lindsay MR, Hernandez-Deviez DJ, Hadzic G, McCluskey A, Bashir R, Liu L, Pilch P, McMahon H, Robinson PJ, Hancock JF, Mayor S, Parton RG. Howes MT, et al. J Cell Biol. 2010 Aug 23;190(4):675-91. doi: 10.1083/jcb.201002119. Epub 2010 Aug 16. J Cell Biol. 2010. PMID: 20713605 Free PMC article. - Polyubiquitinated proteins, proteasome, and glycogen characterize the particle-rich cytoplasmic structure (PaCS) of neoplastic and fetal cells.
Necchi V, Sommi P, Vitali A, Vanoli A, Savoia A, Ricci V, Solcia E. Necchi V, et al. Histochem Cell Biol. 2014 May;141(5):483-97. doi: 10.1007/s00418-014-1202-5. Epub 2014 Mar 1. Histochem Cell Biol. 2014. PMID: 24577783 - Use of larvae of the wax moth Galleria mellonella as an in vivo model to study the virulence of Helicobacter pylori.
Giannouli M, Palatucci AT, Rubino V, Ruggiero G, Romano M, Triassi M, Ricci V, Zarrilli R. Giannouli M, et al. BMC Microbiol. 2014 Aug 27;14:228. doi: 10.1186/s12866-014-0228-0. BMC Microbiol. 2014. PMID: 25170542 Free PMC article.
References
- Abrami L, Fivaz M, Decroly E, Seidah NG, Jean F, Thomas G, Leppla SH, Buckley JT, van der Goot FG. The pore-forming toxin proaerolysin is activated by furin. J Biol Chem. 1998a;273:32656–32661. - PubMed
- Abrami L, Fivaz M, van der Goot FG. Adventures of a pore-forming toxin at the target cell surface. Trends Microbiol. 2000;8:168–172. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous