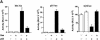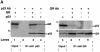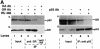Negative cross-talk between p53 and the glucocorticoid receptor and its role in neuroblastoma cells - PubMed (original) (raw)
Negative cross-talk between p53 and the glucocorticoid receptor and its role in neuroblastoma cells
S Sengupta et al. EMBO J. 2000.
Abstract
The tumour suppressor p53 and the glucocorticoid receptor (GR) respond to different types of stress. We found that dexamethasone-activated endogenous and exogenous GR inhibit p53-dependent functions, including transactivation, up- (Bax and p21(WAF1/CIP1)) and down- (Bcl2) regulation of endogenous genes, cell cycle arrest and apoptosis. GR forms a complex with p53 in vivo, resulting in cytoplasmic sequestration of both p53 and GR. In neuroblastoma (NB) cells, cytoplasmic retention and inactivation of wild-type p53 involves GR. p53 and GR form a complex that is dissociated by GR antagonists, resulting in accumulation of p53 in the nucleus, activation of p53-responsive genes, growth arrest and apoptosis. These results suggest that molecules that efficiently disrupt GR-p53 interactions would have a therapeutic potential for the treatment of neuroblastoma and perhaps other diseases in which p53 is sequestered by GR.
Figures
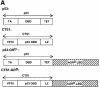
Fig. 1. DITS have attenuated transactivation and growth suppressive functions. (A) Schemes. hGRD-LBD was fused to the C-terminal end of p53 or CTS1 (Conseiller et al., 1998) to generate p53–GRD and CTS–GRD, respectively. CTS1, chimeric tumour suppressor 1; VP16, herpes simplex virus 1 VP16 transcription activation domain; DBD, DNA binding domain; LZ, leucine zipper; LBD, ligand binding domain; TET, tetramerization domain of p53; TA, transactivation domain of p53; hGR, human glucocorticoid receptor. (B) HSC-2 cells were transfected with GRD, p53, p53–GRD, CTS1 or CTS1–GRD expression vectors (250 and 500 ng/ml), the p53-responsive reporter (Bax–luc), and CMV lacZ (500 ng/ml) to measure transfection efficiencies. Activity represents the luciferase values measured 24 h after the end of transfection and corrected for transfection efficiency. The values were similar in three independent transfections. (C) Transfections were carried out as in (B) using 500 ng/ml of the expression plasmids and 500 ng/ml of pEGFP. Cell lysates (50 µg) were analysed by 10% SDS–PAGE and western blotting with (a) a monoclonal antibody that recognizes the DBD of p53 (pAb 240) or (b) anti-GFP. MW = molecular weight markers. Lane 1, mock transfection; lane 2, p53; lane 3, CTS1; lane 4, p53–GRD; lane 5, p53–GRD + Dex; lane 6, CTS1–GRD; lane 7, CTS1–GRD + Dex.
Fig. 1. DITS have attenuated transactivation and growth suppressive functions. (A) Schemes. hGRD-LBD was fused to the C-terminal end of p53 or CTS1 (Conseiller et al., 1998) to generate p53–GRD and CTS–GRD, respectively. CTS1, chimeric tumour suppressor 1; VP16, herpes simplex virus 1 VP16 transcription activation domain; DBD, DNA binding domain; LZ, leucine zipper; LBD, ligand binding domain; TET, tetramerization domain of p53; TA, transactivation domain of p53; hGR, human glucocorticoid receptor. (B) HSC-2 cells were transfected with GRD, p53, p53–GRD, CTS1 or CTS1–GRD expression vectors (250 and 500 ng/ml), the p53-responsive reporter (Bax–luc), and CMV lacZ (500 ng/ml) to measure transfection efficiencies. Activity represents the luciferase values measured 24 h after the end of transfection and corrected for transfection efficiency. The values were similar in three independent transfections. (C) Transfections were carried out as in (B) using 500 ng/ml of the expression plasmids and 500 ng/ml of pEGFP. Cell lysates (50 µg) were analysed by 10% SDS–PAGE and western blotting with (a) a monoclonal antibody that recognizes the DBD of p53 (pAb 240) or (b) anti-GFP. MW = molecular weight markers. Lane 1, mock transfection; lane 2, p53; lane 3, CTS1; lane 4, p53–GRD; lane 5, p53–GRD + Dex; lane 6, CTS1–GRD; lane 7, CTS1–GRD + Dex.
Fig. 1. DITS have attenuated transactivation and growth suppressive functions. (A) Schemes. hGRD-LBD was fused to the C-terminal end of p53 or CTS1 (Conseiller et al., 1998) to generate p53–GRD and CTS–GRD, respectively. CTS1, chimeric tumour suppressor 1; VP16, herpes simplex virus 1 VP16 transcription activation domain; DBD, DNA binding domain; LZ, leucine zipper; LBD, ligand binding domain; TET, tetramerization domain of p53; TA, transactivation domain of p53; hGR, human glucocorticoid receptor. (B) HSC-2 cells were transfected with GRD, p53, p53–GRD, CTS1 or CTS1–GRD expression vectors (250 and 500 ng/ml), the p53-responsive reporter (Bax–luc), and CMV lacZ (500 ng/ml) to measure transfection efficiencies. Activity represents the luciferase values measured 24 h after the end of transfection and corrected for transfection efficiency. The values were similar in three independent transfections. (C) Transfections were carried out as in (B) using 500 ng/ml of the expression plasmids and 500 ng/ml of pEGFP. Cell lysates (50 µg) were analysed by 10% SDS–PAGE and western blotting with (a) a monoclonal antibody that recognizes the DBD of p53 (pAb 240) or (b) anti-GFP. MW = molecular weight markers. Lane 1, mock transfection; lane 2, p53; lane 3, CTS1; lane 4, p53–GRD; lane 5, p53–GRD + Dex; lane 6, CTS1–GRD; lane 7, CTS1–GRD + Dex.
Fig. 2. The human glucocorticoid receptor and p53 inhibit each other’s transactivation properties. (A) HSC-2 cells were transfected with increasing concentrations of either p53 or GR with a constant amount of p53, a minimal p53 reporter (p53 CON) and CMV lacZ to measure transfection efficiency. Vehicle alone (ethanol, –), Cort (10–7 M), Dex (10–7 M) or RU-486 (10–7 M) were added as indicated. Lane 1, mock transfection; lane 2, + Cort; lane 3, + Dex; lane 4, + RU-486; lanes 5–7, p53 (125, 250, 500 ng/ml); lanes 8–10, p53 (125, 250, 500 ng/ml) + Cort; lanes 11–13, p53 (125, 250, 500 ng/ml) + Dex; lanes 14–16, p53 (125, 250, 500 ng/ml) + RU-486; lane 17, p53 (500 ng/ml); lanes 18–20, p53 (500 ng/ml) + GR (250, 500, 1250 ng/ml); lanes 21–23, p53 (500 ng/ml) + GR (250, 500, 1250 ng/ml) + Cort; lanes 24–26, p53 (500 ng/ml) + GR (250, 500, 1250 ng/ml) + Dex; lanes 27–29, p53 (500 ng/ml) + GR (250, 500, 1250 ng/ml) + RU-486. Luciferase activity was measured 24 h post-transfection and corrected for transfection efficiency. The values shown are from four independent experiments. p53 RE, p53-responsive element. TATA, minimal promoter element. (B) Transfections were carried out as in (A), except that 500 ng/ml of pEGFP was included to control for transfection efficiency. Cell lysates (50 µg) were analysed by 10% SDS–PAGE and western blotting with antibodies to: (a) p53 (DO-1), (b) GR (E-20 and P-20) and (c and d) GFP. Lanes 1 and 8, mock transfection with vehicle alone (ethanol); lanes 2–4, p53 (125, 250, 500 ng/ml) with vehicle alone; lanes 5–7, p53 (500 ng/ml) and GR (250, 500 and 1250 ng/ml) + Dex; lanes 9–11, GR (250, 500 and 1250 ng/ml) + Dex. (C) HSC-2 cells were transfected with increasing concentrations of the GR expression vector alone or with a constant amount of the p53 expression vector, the GR reporter (GRE–tk–luc) and CMV lacZ to measure transfection efficiency. Twenty-four hours post-transfection luciferase activities were measured and corrected for transfection efficiencies. The values were similar in three independent experiments. Lane 1, mock transfection; lane 2, mock + Dex; lanes 3–5, GR (250, 500, 1250 ng/ml); lanes 6–8, (250, 500, 1250 ng/ml) + Dex; lanes 9–11, (250, 500, 1250 ng/ml) + p53 (500 ng/ml). –, vehicle alone; +, 10–7 M Dex; GRE, glucocorticoid response element; tk, HSV tk minimal promoter. (D) (a) Transfections with expression vectors for GR or ASGR (500, 1250 ng/ml), alone or in combination, the GRE–tk–luc reporter and CMV lacZ for transfection efficiency. Luciferase was measured 24 h post-transfection and corrected for transfection efficiency. The values are from two independent experiments. Lane 1, mock transfection; lane 2, mock + Dex; lanes 3 and 4, GR (500, 1250 ng/ml); lanes 5 and 6, GR (500, 1250 ng/ml) + Dex; lanes 7 and 8, GR (1250 ng/ml) + ASGR (500, 1250 ng/ml) + Dex; lanes 9 and 10, GR (1250 ng/ml) + ASGR (500, 1250 ng/ml). (b) Cell lysates (50 µg) were prepared 48 h post-transfection and analysed by 10% SDS–PAGE and western blotting with antibodies to TBP (3G3) or GR (E-20 and P-20). Lane 1, untransfected; lane 2, ASGR; lane 3, GR. –, vehicle alone; +, 10–7 M Dex. (E) Transfections contained expression vectors for p53 (250 ng/ml), ASGR (1250 ng/ml), the p53 CON reporter and CMV lacZ to measure transfection efficiency. Luciferase activities were measured after 48 h, corrected for transfection efficiency and expressed as fold induction relative to the control (reporter in the absence of p53). The values represented are from two independent experiments. –, vehicle alone; +, 10–7 M Dex.
Fig. 2. The human glucocorticoid receptor and p53 inhibit each other’s transactivation properties. (A) HSC-2 cells were transfected with increasing concentrations of either p53 or GR with a constant amount of p53, a minimal p53 reporter (p53 CON) and CMV lacZ to measure transfection efficiency. Vehicle alone (ethanol, –), Cort (10–7 M), Dex (10–7 M) or RU-486 (10–7 M) were added as indicated. Lane 1, mock transfection; lane 2, + Cort; lane 3, + Dex; lane 4, + RU-486; lanes 5–7, p53 (125, 250, 500 ng/ml); lanes 8–10, p53 (125, 250, 500 ng/ml) + Cort; lanes 11–13, p53 (125, 250, 500 ng/ml) + Dex; lanes 14–16, p53 (125, 250, 500 ng/ml) + RU-486; lane 17, p53 (500 ng/ml); lanes 18–20, p53 (500 ng/ml) + GR (250, 500, 1250 ng/ml); lanes 21–23, p53 (500 ng/ml) + GR (250, 500, 1250 ng/ml) + Cort; lanes 24–26, p53 (500 ng/ml) + GR (250, 500, 1250 ng/ml) + Dex; lanes 27–29, p53 (500 ng/ml) + GR (250, 500, 1250 ng/ml) + RU-486. Luciferase activity was measured 24 h post-transfection and corrected for transfection efficiency. The values shown are from four independent experiments. p53 RE, p53-responsive element. TATA, minimal promoter element. (B) Transfections were carried out as in (A), except that 500 ng/ml of pEGFP was included to control for transfection efficiency. Cell lysates (50 µg) were analysed by 10% SDS–PAGE and western blotting with antibodies to: (a) p53 (DO-1), (b) GR (E-20 and P-20) and (c and d) GFP. Lanes 1 and 8, mock transfection with vehicle alone (ethanol); lanes 2–4, p53 (125, 250, 500 ng/ml) with vehicle alone; lanes 5–7, p53 (500 ng/ml) and GR (250, 500 and 1250 ng/ml) + Dex; lanes 9–11, GR (250, 500 and 1250 ng/ml) + Dex. (C) HSC-2 cells were transfected with increasing concentrations of the GR expression vector alone or with a constant amount of the p53 expression vector, the GR reporter (GRE–tk–luc) and CMV lacZ to measure transfection efficiency. Twenty-four hours post-transfection luciferase activities were measured and corrected for transfection efficiencies. The values were similar in three independent experiments. Lane 1, mock transfection; lane 2, mock + Dex; lanes 3–5, GR (250, 500, 1250 ng/ml); lanes 6–8, (250, 500, 1250 ng/ml) + Dex; lanes 9–11, (250, 500, 1250 ng/ml) + p53 (500 ng/ml). –, vehicle alone; +, 10–7 M Dex; GRE, glucocorticoid response element; tk, HSV tk minimal promoter. (D) (a) Transfections with expression vectors for GR or ASGR (500, 1250 ng/ml), alone or in combination, the GRE–tk–luc reporter and CMV lacZ for transfection efficiency. Luciferase was measured 24 h post-transfection and corrected for transfection efficiency. The values are from two independent experiments. Lane 1, mock transfection; lane 2, mock + Dex; lanes 3 and 4, GR (500, 1250 ng/ml); lanes 5 and 6, GR (500, 1250 ng/ml) + Dex; lanes 7 and 8, GR (1250 ng/ml) + ASGR (500, 1250 ng/ml) + Dex; lanes 9 and 10, GR (1250 ng/ml) + ASGR (500, 1250 ng/ml). (b) Cell lysates (50 µg) were prepared 48 h post-transfection and analysed by 10% SDS–PAGE and western blotting with antibodies to TBP (3G3) or GR (E-20 and P-20). Lane 1, untransfected; lane 2, ASGR; lane 3, GR. –, vehicle alone; +, 10–7 M Dex. (E) Transfections contained expression vectors for p53 (250 ng/ml), ASGR (1250 ng/ml), the p53 CON reporter and CMV lacZ to measure transfection efficiency. Luciferase activities were measured after 48 h, corrected for transfection efficiency and expressed as fold induction relative to the control (reporter in the absence of p53). The values represented are from two independent experiments. –, vehicle alone; +, 10–7 M Dex.
Fig. 2. The human glucocorticoid receptor and p53 inhibit each other’s transactivation properties. (A) HSC-2 cells were transfected with increasing concentrations of either p53 or GR with a constant amount of p53, a minimal p53 reporter (p53 CON) and CMV lacZ to measure transfection efficiency. Vehicle alone (ethanol, –), Cort (10–7 M), Dex (10–7 M) or RU-486 (10–7 M) were added as indicated. Lane 1, mock transfection; lane 2, + Cort; lane 3, + Dex; lane 4, + RU-486; lanes 5–7, p53 (125, 250, 500 ng/ml); lanes 8–10, p53 (125, 250, 500 ng/ml) + Cort; lanes 11–13, p53 (125, 250, 500 ng/ml) + Dex; lanes 14–16, p53 (125, 250, 500 ng/ml) + RU-486; lane 17, p53 (500 ng/ml); lanes 18–20, p53 (500 ng/ml) + GR (250, 500, 1250 ng/ml); lanes 21–23, p53 (500 ng/ml) + GR (250, 500, 1250 ng/ml) + Cort; lanes 24–26, p53 (500 ng/ml) + GR (250, 500, 1250 ng/ml) + Dex; lanes 27–29, p53 (500 ng/ml) + GR (250, 500, 1250 ng/ml) + RU-486. Luciferase activity was measured 24 h post-transfection and corrected for transfection efficiency. The values shown are from four independent experiments. p53 RE, p53-responsive element. TATA, minimal promoter element. (B) Transfections were carried out as in (A), except that 500 ng/ml of pEGFP was included to control for transfection efficiency. Cell lysates (50 µg) were analysed by 10% SDS–PAGE and western blotting with antibodies to: (a) p53 (DO-1), (b) GR (E-20 and P-20) and (c and d) GFP. Lanes 1 and 8, mock transfection with vehicle alone (ethanol); lanes 2–4, p53 (125, 250, 500 ng/ml) with vehicle alone; lanes 5–7, p53 (500 ng/ml) and GR (250, 500 and 1250 ng/ml) + Dex; lanes 9–11, GR (250, 500 and 1250 ng/ml) + Dex. (C) HSC-2 cells were transfected with increasing concentrations of the GR expression vector alone or with a constant amount of the p53 expression vector, the GR reporter (GRE–tk–luc) and CMV lacZ to measure transfection efficiency. Twenty-four hours post-transfection luciferase activities were measured and corrected for transfection efficiencies. The values were similar in three independent experiments. Lane 1, mock transfection; lane 2, mock + Dex; lanes 3–5, GR (250, 500, 1250 ng/ml); lanes 6–8, (250, 500, 1250 ng/ml) + Dex; lanes 9–11, (250, 500, 1250 ng/ml) + p53 (500 ng/ml). –, vehicle alone; +, 10–7 M Dex; GRE, glucocorticoid response element; tk, HSV tk minimal promoter. (D) (a) Transfections with expression vectors for GR or ASGR (500, 1250 ng/ml), alone or in combination, the GRE–tk–luc reporter and CMV lacZ for transfection efficiency. Luciferase was measured 24 h post-transfection and corrected for transfection efficiency. The values are from two independent experiments. Lane 1, mock transfection; lane 2, mock + Dex; lanes 3 and 4, GR (500, 1250 ng/ml); lanes 5 and 6, GR (500, 1250 ng/ml) + Dex; lanes 7 and 8, GR (1250 ng/ml) + ASGR (500, 1250 ng/ml) + Dex; lanes 9 and 10, GR (1250 ng/ml) + ASGR (500, 1250 ng/ml). (b) Cell lysates (50 µg) were prepared 48 h post-transfection and analysed by 10% SDS–PAGE and western blotting with antibodies to TBP (3G3) or GR (E-20 and P-20). Lane 1, untransfected; lane 2, ASGR; lane 3, GR. –, vehicle alone; +, 10–7 M Dex. (E) Transfections contained expression vectors for p53 (250 ng/ml), ASGR (1250 ng/ml), the p53 CON reporter and CMV lacZ to measure transfection efficiency. Luciferase activities were measured after 48 h, corrected for transfection efficiency and expressed as fold induction relative to the control (reporter in the absence of p53). The values represented are from two independent experiments. –, vehicle alone; +, 10–7 M Dex.
Fig. 2. The human glucocorticoid receptor and p53 inhibit each other’s transactivation properties. (A) HSC-2 cells were transfected with increasing concentrations of either p53 or GR with a constant amount of p53, a minimal p53 reporter (p53 CON) and CMV lacZ to measure transfection efficiency. Vehicle alone (ethanol, –), Cort (10–7 M), Dex (10–7 M) or RU-486 (10–7 M) were added as indicated. Lane 1, mock transfection; lane 2, + Cort; lane 3, + Dex; lane 4, + RU-486; lanes 5–7, p53 (125, 250, 500 ng/ml); lanes 8–10, p53 (125, 250, 500 ng/ml) + Cort; lanes 11–13, p53 (125, 250, 500 ng/ml) + Dex; lanes 14–16, p53 (125, 250, 500 ng/ml) + RU-486; lane 17, p53 (500 ng/ml); lanes 18–20, p53 (500 ng/ml) + GR (250, 500, 1250 ng/ml); lanes 21–23, p53 (500 ng/ml) + GR (250, 500, 1250 ng/ml) + Cort; lanes 24–26, p53 (500 ng/ml) + GR (250, 500, 1250 ng/ml) + Dex; lanes 27–29, p53 (500 ng/ml) + GR (250, 500, 1250 ng/ml) + RU-486. Luciferase activity was measured 24 h post-transfection and corrected for transfection efficiency. The values shown are from four independent experiments. p53 RE, p53-responsive element. TATA, minimal promoter element. (B) Transfections were carried out as in (A), except that 500 ng/ml of pEGFP was included to control for transfection efficiency. Cell lysates (50 µg) were analysed by 10% SDS–PAGE and western blotting with antibodies to: (a) p53 (DO-1), (b) GR (E-20 and P-20) and (c and d) GFP. Lanes 1 and 8, mock transfection with vehicle alone (ethanol); lanes 2–4, p53 (125, 250, 500 ng/ml) with vehicle alone; lanes 5–7, p53 (500 ng/ml) and GR (250, 500 and 1250 ng/ml) + Dex; lanes 9–11, GR (250, 500 and 1250 ng/ml) + Dex. (C) HSC-2 cells were transfected with increasing concentrations of the GR expression vector alone or with a constant amount of the p53 expression vector, the GR reporter (GRE–tk–luc) and CMV lacZ to measure transfection efficiency. Twenty-four hours post-transfection luciferase activities were measured and corrected for transfection efficiencies. The values were similar in three independent experiments. Lane 1, mock transfection; lane 2, mock + Dex; lanes 3–5, GR (250, 500, 1250 ng/ml); lanes 6–8, (250, 500, 1250 ng/ml) + Dex; lanes 9–11, (250, 500, 1250 ng/ml) + p53 (500 ng/ml). –, vehicle alone; +, 10–7 M Dex; GRE, glucocorticoid response element; tk, HSV tk minimal promoter. (D) (a) Transfections with expression vectors for GR or ASGR (500, 1250 ng/ml), alone or in combination, the GRE–tk–luc reporter and CMV lacZ for transfection efficiency. Luciferase was measured 24 h post-transfection and corrected for transfection efficiency. The values are from two independent experiments. Lane 1, mock transfection; lane 2, mock + Dex; lanes 3 and 4, GR (500, 1250 ng/ml); lanes 5 and 6, GR (500, 1250 ng/ml) + Dex; lanes 7 and 8, GR (1250 ng/ml) + ASGR (500, 1250 ng/ml) + Dex; lanes 9 and 10, GR (1250 ng/ml) + ASGR (500, 1250 ng/ml). (b) Cell lysates (50 µg) were prepared 48 h post-transfection and analysed by 10% SDS–PAGE and western blotting with antibodies to TBP (3G3) or GR (E-20 and P-20). Lane 1, untransfected; lane 2, ASGR; lane 3, GR. –, vehicle alone; +, 10–7 M Dex. (E) Transfections contained expression vectors for p53 (250 ng/ml), ASGR (1250 ng/ml), the p53 CON reporter and CMV lacZ to measure transfection efficiency. Luciferase activities were measured after 48 h, corrected for transfection efficiency and expressed as fold induction relative to the control (reporter in the absence of p53). The values represented are from two independent experiments. –, vehicle alone; +, 10–7 M Dex.
Fig. 2. The human glucocorticoid receptor and p53 inhibit each other’s transactivation properties. (A) HSC-2 cells were transfected with increasing concentrations of either p53 or GR with a constant amount of p53, a minimal p53 reporter (p53 CON) and CMV lacZ to measure transfection efficiency. Vehicle alone (ethanol, –), Cort (10–7 M), Dex (10–7 M) or RU-486 (10–7 M) were added as indicated. Lane 1, mock transfection; lane 2, + Cort; lane 3, + Dex; lane 4, + RU-486; lanes 5–7, p53 (125, 250, 500 ng/ml); lanes 8–10, p53 (125, 250, 500 ng/ml) + Cort; lanes 11–13, p53 (125, 250, 500 ng/ml) + Dex; lanes 14–16, p53 (125, 250, 500 ng/ml) + RU-486; lane 17, p53 (500 ng/ml); lanes 18–20, p53 (500 ng/ml) + GR (250, 500, 1250 ng/ml); lanes 21–23, p53 (500 ng/ml) + GR (250, 500, 1250 ng/ml) + Cort; lanes 24–26, p53 (500 ng/ml) + GR (250, 500, 1250 ng/ml) + Dex; lanes 27–29, p53 (500 ng/ml) + GR (250, 500, 1250 ng/ml) + RU-486. Luciferase activity was measured 24 h post-transfection and corrected for transfection efficiency. The values shown are from four independent experiments. p53 RE, p53-responsive element. TATA, minimal promoter element. (B) Transfections were carried out as in (A), except that 500 ng/ml of pEGFP was included to control for transfection efficiency. Cell lysates (50 µg) were analysed by 10% SDS–PAGE and western blotting with antibodies to: (a) p53 (DO-1), (b) GR (E-20 and P-20) and (c and d) GFP. Lanes 1 and 8, mock transfection with vehicle alone (ethanol); lanes 2–4, p53 (125, 250, 500 ng/ml) with vehicle alone; lanes 5–7, p53 (500 ng/ml) and GR (250, 500 and 1250 ng/ml) + Dex; lanes 9–11, GR (250, 500 and 1250 ng/ml) + Dex. (C) HSC-2 cells were transfected with increasing concentrations of the GR expression vector alone or with a constant amount of the p53 expression vector, the GR reporter (GRE–tk–luc) and CMV lacZ to measure transfection efficiency. Twenty-four hours post-transfection luciferase activities were measured and corrected for transfection efficiencies. The values were similar in three independent experiments. Lane 1, mock transfection; lane 2, mock + Dex; lanes 3–5, GR (250, 500, 1250 ng/ml); lanes 6–8, (250, 500, 1250 ng/ml) + Dex; lanes 9–11, (250, 500, 1250 ng/ml) + p53 (500 ng/ml). –, vehicle alone; +, 10–7 M Dex; GRE, glucocorticoid response element; tk, HSV tk minimal promoter. (D) (a) Transfections with expression vectors for GR or ASGR (500, 1250 ng/ml), alone or in combination, the GRE–tk–luc reporter and CMV lacZ for transfection efficiency. Luciferase was measured 24 h post-transfection and corrected for transfection efficiency. The values are from two independent experiments. Lane 1, mock transfection; lane 2, mock + Dex; lanes 3 and 4, GR (500, 1250 ng/ml); lanes 5 and 6, GR (500, 1250 ng/ml) + Dex; lanes 7 and 8, GR (1250 ng/ml) + ASGR (500, 1250 ng/ml) + Dex; lanes 9 and 10, GR (1250 ng/ml) + ASGR (500, 1250 ng/ml). (b) Cell lysates (50 µg) were prepared 48 h post-transfection and analysed by 10% SDS–PAGE and western blotting with antibodies to TBP (3G3) or GR (E-20 and P-20). Lane 1, untransfected; lane 2, ASGR; lane 3, GR. –, vehicle alone; +, 10–7 M Dex. (E) Transfections contained expression vectors for p53 (250 ng/ml), ASGR (1250 ng/ml), the p53 CON reporter and CMV lacZ to measure transfection efficiency. Luciferase activities were measured after 48 h, corrected for transfection efficiency and expressed as fold induction relative to the control (reporter in the absence of p53). The values represented are from two independent experiments. –, vehicle alone; +, 10–7 M Dex.
Fig. 3. p53-responsive genes are modulated by Dex. (A) HSC-2 cells were transfected with an expression vector for p53 (filled bars) or the corresponding empty vector (open bars), and reporters containing the natural p53-responsive promoters bax, p21WAF1/CIP1 and bcl2 linked to luciferase, and CMV lacZ to measure transfection efficiency. Luciferase activity was assayed 24 h post-transfection and corrected for transfection efficiency. The values are from two independent experiments. (B) Transfections were carried out as in (A). Additional plates with Cort (10–7 M) were included. pEGFP (500 ng/ml) was used to measure transfection efficiency. Cell lysates (50 µg) were analysed by SDS–PAGE and western blotting with antibodies to: (a and f) Bax (N-20, polyclonal); (b and g) p21WAF1/CIP1 (1WA-IC581, monoclonal); (c) bcl2 (100, monoclonal); (d and h) p53 (DO-1, monoclonal); (e and i) GFP (monoclonal). Lanes 1 and 5, mock transfection; lane 2, mock + Dex; lanes 3 and 7, p53; lane 4, p53 + Dex; lane 6, Cort; lane 8, p53 + Cort. –, vehicle alone; +, 10–7 M Dex.
Fig. 3. p53-responsive genes are modulated by Dex. (A) HSC-2 cells were transfected with an expression vector for p53 (filled bars) or the corresponding empty vector (open bars), and reporters containing the natural p53-responsive promoters bax, p21WAF1/CIP1 and bcl2 linked to luciferase, and CMV lacZ to measure transfection efficiency. Luciferase activity was assayed 24 h post-transfection and corrected for transfection efficiency. The values are from two independent experiments. (B) Transfections were carried out as in (A). Additional plates with Cort (10–7 M) were included. pEGFP (500 ng/ml) was used to measure transfection efficiency. Cell lysates (50 µg) were analysed by SDS–PAGE and western blotting with antibodies to: (a and f) Bax (N-20, polyclonal); (b and g) p21WAF1/CIP1 (1WA-IC581, monoclonal); (c) bcl2 (100, monoclonal); (d and h) p53 (DO-1, monoclonal); (e and i) GFP (monoclonal). Lanes 1 and 5, mock transfection; lane 2, mock + Dex; lanes 3 and 7, p53; lane 4, p53 + Dex; lane 6, Cort; lane 8, p53 + Cort. –, vehicle alone; +, 10–7 M Dex.
Fig. 4. GR inhibits p53-mediated apoptosis and growth suppression. (A) Cells were transfected with expression vectors for p53 (250 ng/ml) and/or GR (1250 ng/ml) in the presence of either vehicle (ethanol) or 10–7 Dex. Cell lysates (50 ng) prepared 16 h post-transfection were analysed by SDS–PAGE and western blotting with antibodies to (a) PARP (polyclonal) and (b) TBP (3G3, monoclonal). Lane 1, mock transfection; lane 2, p53 + GR + Dex; lane 3, p53; lane 4, p53 + GR. –, vehicle; +, 10–7 M Dex. The PARP cleavage product (CP) was scanned and the values are the amount of CP relative to p53 alone (lane 3). (B) Cells were transfected with p53 (250 and 500 ng/ml) and/or GR (1250 ng/ml) expression plasmids in the presence of either vehicle (ethanol) or 10–7 M Dex. Low molecular weight DNA was extracted 24 h post-transfection, radiolabelled, run on 1.5% TBE agarose gels, transferred to nylon membranes and visualized by autoradiography. Lane 1, mock transfection with vehicle alone (–); lane 2, mock + Dex; lane 3, GR; lane 4, GR + Dex; lanes 5 and 6, p53 (250, 500 ng/ml); lanes 7 and 8; p53 (250, 500 ng/ml) + GR + Dex; lanes 9 and 10, p53 (250, 500 ng/ml) + GR; lanes 11 and 12, p53 (250, 500 ng/ml) + Dex. (C) Transfections as in (A) with expression plasmids for p53 (500 ng/ml), GR (1250 ng/ml), CD20 (4 µg/ml, to select transfected cells), and vehicle (ethanol) or Dex. Twenty-four hours post-transfection, the cells were harvested and analysed by flow cytometry. Transfections were duplicated and the whole experiment was repeated. The values (with standard deviation) are from four experimental points. The percentage of transfected cells in each stage of the cell cycle was substracted from the control transfected with empty vector (control). Hence the control (with no change) is not visible. The cell cycle distribution of the control was 10% sub-G0, 36% G0–G1, 44% S, 10% G2–M. (D) Clonogenic assays with an expression vector for p53 and the corresponding empty vector (CMV NeoBam) with vehicle (–) or 10–7 M Dex (+). Triplicate independent transfections were carried out for each point. The values are from two independent experiments.
Fig. 4. GR inhibits p53-mediated apoptosis and growth suppression. (A) Cells were transfected with expression vectors for p53 (250 ng/ml) and/or GR (1250 ng/ml) in the presence of either vehicle (ethanol) or 10–7 Dex. Cell lysates (50 ng) prepared 16 h post-transfection were analysed by SDS–PAGE and western blotting with antibodies to (a) PARP (polyclonal) and (b) TBP (3G3, monoclonal). Lane 1, mock transfection; lane 2, p53 + GR + Dex; lane 3, p53; lane 4, p53 + GR. –, vehicle; +, 10–7 M Dex. The PARP cleavage product (CP) was scanned and the values are the amount of CP relative to p53 alone (lane 3). (B) Cells were transfected with p53 (250 and 500 ng/ml) and/or GR (1250 ng/ml) expression plasmids in the presence of either vehicle (ethanol) or 10–7 M Dex. Low molecular weight DNA was extracted 24 h post-transfection, radiolabelled, run on 1.5% TBE agarose gels, transferred to nylon membranes and visualized by autoradiography. Lane 1, mock transfection with vehicle alone (–); lane 2, mock + Dex; lane 3, GR; lane 4, GR + Dex; lanes 5 and 6, p53 (250, 500 ng/ml); lanes 7 and 8; p53 (250, 500 ng/ml) + GR + Dex; lanes 9 and 10, p53 (250, 500 ng/ml) + GR; lanes 11 and 12, p53 (250, 500 ng/ml) + Dex. (C) Transfections as in (A) with expression plasmids for p53 (500 ng/ml), GR (1250 ng/ml), CD20 (4 µg/ml, to select transfected cells), and vehicle (ethanol) or Dex. Twenty-four hours post-transfection, the cells were harvested and analysed by flow cytometry. Transfections were duplicated and the whole experiment was repeated. The values (with standard deviation) are from four experimental points. The percentage of transfected cells in each stage of the cell cycle was substracted from the control transfected with empty vector (control). Hence the control (with no change) is not visible. The cell cycle distribution of the control was 10% sub-G0, 36% G0–G1, 44% S, 10% G2–M. (D) Clonogenic assays with an expression vector for p53 and the corresponding empty vector (CMV NeoBam) with vehicle (–) or 10–7 M Dex (+). Triplicate independent transfections were carried out for each point. The values are from two independent experiments.
Fig. 4. GR inhibits p53-mediated apoptosis and growth suppression. (A) Cells were transfected with expression vectors for p53 (250 ng/ml) and/or GR (1250 ng/ml) in the presence of either vehicle (ethanol) or 10–7 Dex. Cell lysates (50 ng) prepared 16 h post-transfection were analysed by SDS–PAGE and western blotting with antibodies to (a) PARP (polyclonal) and (b) TBP (3G3, monoclonal). Lane 1, mock transfection; lane 2, p53 + GR + Dex; lane 3, p53; lane 4, p53 + GR. –, vehicle; +, 10–7 M Dex. The PARP cleavage product (CP) was scanned and the values are the amount of CP relative to p53 alone (lane 3). (B) Cells were transfected with p53 (250 and 500 ng/ml) and/or GR (1250 ng/ml) expression plasmids in the presence of either vehicle (ethanol) or 10–7 M Dex. Low molecular weight DNA was extracted 24 h post-transfection, radiolabelled, run on 1.5% TBE agarose gels, transferred to nylon membranes and visualized by autoradiography. Lane 1, mock transfection with vehicle alone (–); lane 2, mock + Dex; lane 3, GR; lane 4, GR + Dex; lanes 5 and 6, p53 (250, 500 ng/ml); lanes 7 and 8; p53 (250, 500 ng/ml) + GR + Dex; lanes 9 and 10, p53 (250, 500 ng/ml) + GR; lanes 11 and 12, p53 (250, 500 ng/ml) + Dex. (C) Transfections as in (A) with expression plasmids for p53 (500 ng/ml), GR (1250 ng/ml), CD20 (4 µg/ml, to select transfected cells), and vehicle (ethanol) or Dex. Twenty-four hours post-transfection, the cells were harvested and analysed by flow cytometry. Transfections were duplicated and the whole experiment was repeated. The values (with standard deviation) are from four experimental points. The percentage of transfected cells in each stage of the cell cycle was substracted from the control transfected with empty vector (control). Hence the control (with no change) is not visible. The cell cycle distribution of the control was 10% sub-G0, 36% G0–G1, 44% S, 10% G2–M. (D) Clonogenic assays with an expression vector for p53 and the corresponding empty vector (CMV NeoBam) with vehicle (–) or 10–7 M Dex (+). Triplicate independent transfections were carried out for each point. The values are from two independent experiments.
Fig. 4. GR inhibits p53-mediated apoptosis and growth suppression. (A) Cells were transfected with expression vectors for p53 (250 ng/ml) and/or GR (1250 ng/ml) in the presence of either vehicle (ethanol) or 10–7 Dex. Cell lysates (50 ng) prepared 16 h post-transfection were analysed by SDS–PAGE and western blotting with antibodies to (a) PARP (polyclonal) and (b) TBP (3G3, monoclonal). Lane 1, mock transfection; lane 2, p53 + GR + Dex; lane 3, p53; lane 4, p53 + GR. –, vehicle; +, 10–7 M Dex. The PARP cleavage product (CP) was scanned and the values are the amount of CP relative to p53 alone (lane 3). (B) Cells were transfected with p53 (250 and 500 ng/ml) and/or GR (1250 ng/ml) expression plasmids in the presence of either vehicle (ethanol) or 10–7 M Dex. Low molecular weight DNA was extracted 24 h post-transfection, radiolabelled, run on 1.5% TBE agarose gels, transferred to nylon membranes and visualized by autoradiography. Lane 1, mock transfection with vehicle alone (–); lane 2, mock + Dex; lane 3, GR; lane 4, GR + Dex; lanes 5 and 6, p53 (250, 500 ng/ml); lanes 7 and 8; p53 (250, 500 ng/ml) + GR + Dex; lanes 9 and 10, p53 (250, 500 ng/ml) + GR; lanes 11 and 12, p53 (250, 500 ng/ml) + Dex. (C) Transfections as in (A) with expression plasmids for p53 (500 ng/ml), GR (1250 ng/ml), CD20 (4 µg/ml, to select transfected cells), and vehicle (ethanol) or Dex. Twenty-four hours post-transfection, the cells were harvested and analysed by flow cytometry. Transfections were duplicated and the whole experiment was repeated. The values (with standard deviation) are from four experimental points. The percentage of transfected cells in each stage of the cell cycle was substracted from the control transfected with empty vector (control). Hence the control (with no change) is not visible. The cell cycle distribution of the control was 10% sub-G0, 36% G0–G1, 44% S, 10% G2–M. (D) Clonogenic assays with an expression vector for p53 and the corresponding empty vector (CMV NeoBam) with vehicle (–) or 10–7 M Dex (+). Triplicate independent transfections were carried out for each point. The values are from two independent experiments.
Fig. 5. p53 and GR interact physically and co-localize in the cell. (A) HSC-2 cells were transfected with p53 (500 ng/ml) and/or GR (1250 ng/ml) expression plasmids, as indicated, in the presence of 10–7 M Dex. Lysates prepared 24 h post-transfection were immunoprecipitated (IP) with either p53 (DO-1, monoclonal) or GR (E-20 and P-20, polyclonal) antibodies (Ab). After the washes, the immunoprecipitates were analysed by 10% SDS–PAGE and western blotting with antibodies against either GR (p53 IP, panel a) or p53 (GR IP, panel c). The efficiency of immunoprecipitation was checked by western blotting with self antibodies (panels b and d). Lane 1, transfected GR, no IP (20% of p53 input); lane 2, mock transfection, IP with p53 Ab; lane 3, transfected GR and p53, IP with p53 Ab; lane 4, transfected p53, IP with p53 Ab; lane 5, transfected p53, no IP (20% of input for GR IP); lane 6, transfected p53 and GR, IP with GR Ab; lane 7, mock transfection, IP with GR Ab; lane 8, transfected GR, IP with GR Ab. (B) HSC-2 (a) or Saos-2 (b) cells were transfected with p53 (500 ng/ml) and/or GR (1250 ng/ml) expression plasmids in the presence of either vehicle (ethanol) or 10–7 M Dex. Twenty-four hours post-transfection the cells were fixed, incubated with antibodies against GR (E-20 and P-20, polyclonal) or p53 (DO-1, monoclonal) and analysed by confocal microscopy. (C) Quantitation of the transfections in (B). At least 500 cells were counted for each transfection. The percentage of cells with the protein in the nucleus (N), the cytoplasm (C) and both (C + N) is shown. (a) GR; (b) GR + Dex; (c) p53; (d) p53 + GR; (e) p53 + GR + Dex; (f) p53 + Dex; (g) p53 + ASGR + Dex. Open bars, GR; filled bars, p53.
Fig. 5. p53 and GR interact physically and co-localize in the cell. (A) HSC-2 cells were transfected with p53 (500 ng/ml) and/or GR (1250 ng/ml) expression plasmids, as indicated, in the presence of 10–7 M Dex. Lysates prepared 24 h post-transfection were immunoprecipitated (IP) with either p53 (DO-1, monoclonal) or GR (E-20 and P-20, polyclonal) antibodies (Ab). After the washes, the immunoprecipitates were analysed by 10% SDS–PAGE and western blotting with antibodies against either GR (p53 IP, panel a) or p53 (GR IP, panel c). The efficiency of immunoprecipitation was checked by western blotting with self antibodies (panels b and d). Lane 1, transfected GR, no IP (20% of p53 input); lane 2, mock transfection, IP with p53 Ab; lane 3, transfected GR and p53, IP with p53 Ab; lane 4, transfected p53, IP with p53 Ab; lane 5, transfected p53, no IP (20% of input for GR IP); lane 6, transfected p53 and GR, IP with GR Ab; lane 7, mock transfection, IP with GR Ab; lane 8, transfected GR, IP with GR Ab. (B) HSC-2 (a) or Saos-2 (b) cells were transfected with p53 (500 ng/ml) and/or GR (1250 ng/ml) expression plasmids in the presence of either vehicle (ethanol) or 10–7 M Dex. Twenty-four hours post-transfection the cells were fixed, incubated with antibodies against GR (E-20 and P-20, polyclonal) or p53 (DO-1, monoclonal) and analysed by confocal microscopy. (C) Quantitation of the transfections in (B). At least 500 cells were counted for each transfection. The percentage of cells with the protein in the nucleus (N), the cytoplasm (C) and both (C + N) is shown. (a) GR; (b) GR + Dex; (c) p53; (d) p53 + GR; (e) p53 + GR + Dex; (f) p53 + Dex; (g) p53 + ASGR + Dex. Open bars, GR; filled bars, p53.
Fig. 5. p53 and GR interact physically and co-localize in the cell. (A) HSC-2 cells were transfected with p53 (500 ng/ml) and/or GR (1250 ng/ml) expression plasmids, as indicated, in the presence of 10–7 M Dex. Lysates prepared 24 h post-transfection were immunoprecipitated (IP) with either p53 (DO-1, monoclonal) or GR (E-20 and P-20, polyclonal) antibodies (Ab). After the washes, the immunoprecipitates were analysed by 10% SDS–PAGE and western blotting with antibodies against either GR (p53 IP, panel a) or p53 (GR IP, panel c). The efficiency of immunoprecipitation was checked by western blotting with self antibodies (panels b and d). Lane 1, transfected GR, no IP (20% of p53 input); lane 2, mock transfection, IP with p53 Ab; lane 3, transfected GR and p53, IP with p53 Ab; lane 4, transfected p53, IP with p53 Ab; lane 5, transfected p53, no IP (20% of input for GR IP); lane 6, transfected p53 and GR, IP with GR Ab; lane 7, mock transfection, IP with GR Ab; lane 8, transfected GR, IP with GR Ab. (B) HSC-2 (a) or Saos-2 (b) cells were transfected with p53 (500 ng/ml) and/or GR (1250 ng/ml) expression plasmids in the presence of either vehicle (ethanol) or 10–7 M Dex. Twenty-four hours post-transfection the cells were fixed, incubated with antibodies against GR (E-20 and P-20, polyclonal) or p53 (DO-1, monoclonal) and analysed by confocal microscopy. (C) Quantitation of the transfections in (B). At least 500 cells were counted for each transfection. The percentage of cells with the protein in the nucleus (N), the cytoplasm (C) and both (C + N) is shown. (a) GR; (b) GR + Dex; (c) p53; (d) p53 + GR; (e) p53 + GR + Dex; (f) p53 + Dex; (g) p53 + ASGR + Dex. Open bars, GR; filled bars, p53.
Fig. 6. The GR antagonist RU-486 disrupts the GR–p53 complex and increases p53 localization in the nucleus in neuroblastoma cells. (A) IMR 32 cells were grown for 24 h in the presence of vehicle (ethanol) or 10–7 M RU-486. Immunoprecipitates (IP) formed with Ab against p53 (DO-1, monoclonal), HA (12CA5, monoclonal) or GR (E-20 and P-20, polyclonal) were washed, subjected to 10% SDS–PAGE and western blotted with Abs against p53 (GR and HA IP, panel a) or GR (p53 IP, panel c). The efficiency of IP was checked by western blotting with self antibodies (panels b and d). Lane 1, IMR 32 cells with vehicle (–, 5% of the lysate used for GR/HA IP); lane 2, IMR 32 cells with RU-486 (+, 5% of input for GR/HA IP); lane 3, vehicle (–), IP with GR Ab; lane 4, RU-486 (+), IP with GR Ab; lane 5, vehicle (–), IP with HA Ab; lane 6, vehicle (–, 20% of input for p53 IP); lane 7, RU-486 (+, 20% of input for p53 IP); lane 8, vehicle (–), IP with p53 Ab; lane 9, RU-486 (+), IP with p53 Ab. (B) IMR 32 cells were cultured with vehicle (control), RU-486 or Dex, and after 24 h they were fixed, incubated with antibodies against GR (E-20 and p20, polyclonal) or p53 (DO-1, monoclonal) and examined by confocal microscopy. (C) Quantification of (B). For each group ∼1000 cells were counted. (a) control; (b) RU-486; (c) Dex. N, nuclear; C, cytoplasmic; C + N, both cytoplasmic and nuclear.
Fig. 6. The GR antagonist RU-486 disrupts the GR–p53 complex and increases p53 localization in the nucleus in neuroblastoma cells. (A) IMR 32 cells were grown for 24 h in the presence of vehicle (ethanol) or 10–7 M RU-486. Immunoprecipitates (IP) formed with Ab against p53 (DO-1, monoclonal), HA (12CA5, monoclonal) or GR (E-20 and P-20, polyclonal) were washed, subjected to 10% SDS–PAGE and western blotted with Abs against p53 (GR and HA IP, panel a) or GR (p53 IP, panel c). The efficiency of IP was checked by western blotting with self antibodies (panels b and d). Lane 1, IMR 32 cells with vehicle (–, 5% of the lysate used for GR/HA IP); lane 2, IMR 32 cells with RU-486 (+, 5% of input for GR/HA IP); lane 3, vehicle (–), IP with GR Ab; lane 4, RU-486 (+), IP with GR Ab; lane 5, vehicle (–), IP with HA Ab; lane 6, vehicle (–, 20% of input for p53 IP); lane 7, RU-486 (+, 20% of input for p53 IP); lane 8, vehicle (–), IP with p53 Ab; lane 9, RU-486 (+), IP with p53 Ab. (B) IMR 32 cells were cultured with vehicle (control), RU-486 or Dex, and after 24 h they were fixed, incubated with antibodies against GR (E-20 and p20, polyclonal) or p53 (DO-1, monoclonal) and examined by confocal microscopy. (C) Quantification of (B). For each group ∼1000 cells were counted. (a) control; (b) RU-486; (c) Dex. N, nuclear; C, cytoplasmic; C + N, both cytoplasmic and nuclear.
Fig. 6. The GR antagonist RU-486 disrupts the GR–p53 complex and increases p53 localization in the nucleus in neuroblastoma cells. (A) IMR 32 cells were grown for 24 h in the presence of vehicle (ethanol) or 10–7 M RU-486. Immunoprecipitates (IP) formed with Ab against p53 (DO-1, monoclonal), HA (12CA5, monoclonal) or GR (E-20 and P-20, polyclonal) were washed, subjected to 10% SDS–PAGE and western blotted with Abs against p53 (GR and HA IP, panel a) or GR (p53 IP, panel c). The efficiency of IP was checked by western blotting with self antibodies (panels b and d). Lane 1, IMR 32 cells with vehicle (–, 5% of the lysate used for GR/HA IP); lane 2, IMR 32 cells with RU-486 (+, 5% of input for GR/HA IP); lane 3, vehicle (–), IP with GR Ab; lane 4, RU-486 (+), IP with GR Ab; lane 5, vehicle (–), IP with HA Ab; lane 6, vehicle (–, 20% of input for p53 IP); lane 7, RU-486 (+, 20% of input for p53 IP); lane 8, vehicle (–), IP with p53 Ab; lane 9, RU-486 (+), IP with p53 Ab. (B) IMR 32 cells were cultured with vehicle (control), RU-486 or Dex, and after 24 h they were fixed, incubated with antibodies against GR (E-20 and p20, polyclonal) or p53 (DO-1, monoclonal) and examined by confocal microscopy. (C) Quantification of (B). For each group ∼1000 cells were counted. (a) control; (b) RU-486; (c) Dex. N, nuclear; C, cytoplasmic; C + N, both cytoplasmic and nuclear.
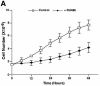
Fig. 7. Effect of GR antagonists on the growth, expression of p53-responsive genes and the cell cycle of IMR 32 neuroblastoma cells. (A) Equal numbers (1.7 × 10–6) of IMR 32 cells were plated in the presence of vehicle (open symbols) or 10–7 M RU-486 (closed symbols). At different times the number of viable cells was counted. The values are from three independent experiments. (B) IMR 32 cells were cultured with vehicle, 10–7 M RU-486 or 10–7 M RU-044 for 24 h. Lysates (100 µg) were analysed by SDS–PAGE and western blotting with antibodies to (a) p53 (DO-1, monoclonal), (b) p21_WAF1/CIP1_ (1WA-IC581, monoclonal), (c) Bax, (N-20, polyclonal), (d) TBP (3G3, monoclonal). Lane 1, cells before ligand addition; lane 2, vehicle (–); lane 3, RU-486 (+); lane 4, RU-044 (+). (C) IMR 32 cells were cultured as in (B) and additionally with mitomycin C (1 µM) as indicated. After 24 h of treatment, the cells were harvested and analysed by flow cytometry. Experimental points were in duplicate and the whole experiment was repeated. The values (with standard deviation) are from four experimental points. The percentage of cells in each stage of the cell cycle was subtracted from the control (2% sub-G0, 39% G0–G1, 48% S, 11% G2–M). The control, with zero change, is not visible.
Fig. 7. Effect of GR antagonists on the growth, expression of p53-responsive genes and the cell cycle of IMR 32 neuroblastoma cells. (A) Equal numbers (1.7 × 10–6) of IMR 32 cells were plated in the presence of vehicle (open symbols) or 10–7 M RU-486 (closed symbols). At different times the number of viable cells was counted. The values are from three independent experiments. (B) IMR 32 cells were cultured with vehicle, 10–7 M RU-486 or 10–7 M RU-044 for 24 h. Lysates (100 µg) were analysed by SDS–PAGE and western blotting with antibodies to (a) p53 (DO-1, monoclonal), (b) p21_WAF1/CIP1_ (1WA-IC581, monoclonal), (c) Bax, (N-20, polyclonal), (d) TBP (3G3, monoclonal). Lane 1, cells before ligand addition; lane 2, vehicle (–); lane 3, RU-486 (+); lane 4, RU-044 (+). (C) IMR 32 cells were cultured as in (B) and additionally with mitomycin C (1 µM) as indicated. After 24 h of treatment, the cells were harvested and analysed by flow cytometry. Experimental points were in duplicate and the whole experiment was repeated. The values (with standard deviation) are from four experimental points. The percentage of cells in each stage of the cell cycle was subtracted from the control (2% sub-G0, 39% G0–G1, 48% S, 11% G2–M). The control, with zero change, is not visible.
Fig. 7. Effect of GR antagonists on the growth, expression of p53-responsive genes and the cell cycle of IMR 32 neuroblastoma cells. (A) Equal numbers (1.7 × 10–6) of IMR 32 cells were plated in the presence of vehicle (open symbols) or 10–7 M RU-486 (closed symbols). At different times the number of viable cells was counted. The values are from three independent experiments. (B) IMR 32 cells were cultured with vehicle, 10–7 M RU-486 or 10–7 M RU-044 for 24 h. Lysates (100 µg) were analysed by SDS–PAGE and western blotting with antibodies to (a) p53 (DO-1, monoclonal), (b) p21_WAF1/CIP1_ (1WA-IC581, monoclonal), (c) Bax, (N-20, polyclonal), (d) TBP (3G3, monoclonal). Lane 1, cells before ligand addition; lane 2, vehicle (–); lane 3, RU-486 (+); lane 4, RU-044 (+). (C) IMR 32 cells were cultured as in (B) and additionally with mitomycin C (1 µM) as indicated. After 24 h of treatment, the cells were harvested and analysed by flow cytometry. Experimental points were in duplicate and the whole experiment was repeated. The values (with standard deviation) are from four experimental points. The percentage of cells in each stage of the cell cycle was subtracted from the control (2% sub-G0, 39% G0–G1, 48% S, 11% G2–M). The control, with zero change, is not visible.
Similar articles
- Ser/Thr protein phosphatase type 5 (PP5) is a negative regulator of glucocorticoid receptor-mediated growth arrest.
Zuo Z, Urban G, Scammell JG, Dean NM, McLean TK, Aragon I, Honkanen RE. Zuo Z, et al. Biochemistry. 1999 Jul 13;38(28):8849-57. doi: 10.1021/bi990842e. Biochemistry. 1999. PMID: 10413457 - Enhancement of p53 activity and inhibition of neural cell proliferation by glucocorticoid receptor activation.
Crochemore C, Michaelidis TM, Fischer D, Loeffler JP, Almeida OF. Crochemore C, et al. FASEB J. 2002 Jun;16(8):761-70. doi: 10.1096/fj.01-0577com. FASEB J. 2002. PMID: 12039857 - Functional p53 is required for triptolide-induced apoptosis and AP-1 and nuclear factor-kappaB activation in gastric cancer cells.
Jiang XH, Wong BC, Lin MC, Zhu GH, Kung HF, Jiang SH, Yang D, Lam SK. Jiang XH, et al. Oncogene. 2001 Nov 29;20(55):8009-18. doi: 10.1038/sj.onc.1204981. Oncogene. 2001. PMID: 11753684 - Cytoplasmic functions of the tumour suppressor p53.
Green DR, Kroemer G. Green DR, et al. Nature. 2009 Apr 30;458(7242):1127-30. doi: 10.1038/nature07986. Nature. 2009. PMID: 19407794 Free PMC article. Review. - p53 and apoptosis: it's not just in the nucleus anymore.
Manfredi JJ. Manfredi JJ. Mol Cell. 2003 Mar;11(3):552-4. doi: 10.1016/s1097-2765(03)00106-0. Mol Cell. 2003. PMID: 12667439 Review.
Cited by
- Glucocorticoid receptor: a harmonizer of cellular plasticity in breast cancer-directs the road towards therapy resistance, metastatic progression and recurrence.
Thakur D, Sengupta D, Mahapatra E, Das S, Sarkar R, Mukherjee S. Thakur D, et al. Cancer Metastasis Rev. 2024 Mar;43(1):481-499. doi: 10.1007/s10555-023-10163-6. Epub 2024 Jan 3. Cancer Metastasis Rev. 2024. PMID: 38170347 Review. - Pilot study suggests DNA methylation of the glucocorticoid receptor gene (NR3C1) is associated with MDMA-assisted therapy treatment response for severe PTSD.
Lewis CR, Tafur J, Spencer S, Green JM, Harrison C, Kelmendi B, Rabin DM, Yehuda R, Yazar-Klosinski B, Cahn BR. Lewis CR, et al. Front Psychiatry. 2023 Feb 6;14:959590. doi: 10.3389/fpsyt.2023.959590. eCollection 2023. Front Psychiatry. 2023. PMID: 36815187 Free PMC article. - Effect of Glucocorticosteroids in Diamond-Blackfan Anaemia: Maybe Not as Elusive as It Seems.
Macečková Z, Kubíčková A, De Sanctis JB, Hajdúch M. Macečková Z, et al. Int J Mol Sci. 2022 Feb 8;23(3):1886. doi: 10.3390/ijms23031886. Int J Mol Sci. 2022. PMID: 35163808 Free PMC article. Review. - Acquired Glucocorticoid Resistance Due to Homologous Glucocorticoid Receptor Downregulation: A Modern Look at an Age-Old Problem.
Spies LL, Verhoog NJD, Louw A. Spies LL, et al. Cells. 2021 Sep 24;10(10):2529. doi: 10.3390/cells10102529. Cells. 2021. PMID: 34685511 Free PMC article. Review. - Combinatorial actions of glucocorticoid and mineralocorticoid stress hormone receptors are required for preventing neurodegeneration of the mouse hippocampus.
Oakley RH, Whirledge SD, Petrillo MG, Riddick NV, Xu X, Moy SS, Cidlowski JA. Oakley RH, et al. Neurobiol Stress. 2021 Jul 21;15:100369. doi: 10.1016/j.ynstr.2021.100369. eCollection 2021 Nov. Neurobiol Stress. 2021. PMID: 34368410 Free PMC article.
References
- Aladjem M.I., Spike,B.T., Rodewald,L.W., Hope,T.J., Klemm,M., Jaenisch,R. and Wahl,G.M. (1998) ES cells do not activate p53-dependent stress responses and undergo p53-independent apoptosis in response to DNA damage. Curr. Biol., 8, 145–155. - PubMed
- An W.G., Kanekal,M., Simon,M.C., Maltepe,E., Blagosklonny,M.V. and Neckers,L.M. (1998) Stabilization of wild-type p53 by hypoxia-inducible factor 1α. Nature, 392, 405–408. - PubMed
- Barnes P.J. and Adcock,I. (1993) Anti-inflammatory actions of steroids: molecular mechanisms. Trends Pharmacol. Sci., 14, 436–441. - PubMed
- Baulieu E.E. (1997) RU 486 (mifepristone). A short overview of its mechanisms of action and clinical uses at the end of 1996. Ann. NY Acad. Sci., 828, 47–58. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous