p53-inducible wip1 phosphatase mediates a negative feedback regulation of p38 MAPK-p53 signaling in response to UV radiation - PubMed (original) (raw)
p53-inducible wip1 phosphatase mediates a negative feedback regulation of p38 MAPK-p53 signaling in response to UV radiation
M Takekawa et al. EMBO J. 2000.
Abstract
The stress-responsive p38 MAPK, when activated by genotoxic stresses such as UV radiation, enhances p53 activity by phosphorylation and leads to cell cycle arrest or apoptosis. Here we report that a member of the protein phosphatase type 2C family, Wip1, has a role in down-regulating p38-p53 signaling during the recovery phase of the damaged cells. Wip1 was originally identified as a gene whose expression is induced following gamma or UV radiation in a p53-dependent manner. We found that Wip1 is also inducible by other environmental stresses, such as anisomycin, H(2)O(2) and methyl methane sulfonate. UV-induction of Wip1 requires p38 activity in addition to the wild-type p53. Wip1 selectively inactivates p38 by specific dephosphorylation of its conserved threonine residue. Furthermore, Wip1 expression attenuates UV-induced p53 phosphorylation at Ser33 and Ser46, residues previously reported to be phosphorylated by p38. Wip1 expression also suppresses both p53-mediated transcription and apoptosis in response to UV radiation. These results suggest that p53-dependent expression of Wip1 mediates a negative feedback regulation of p38-p53 signaling and contributes to suppression of the UV-induced apoptosis.
Figures
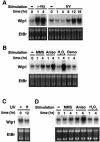
Fig. 1. Northern blot analysis of Wip1 expression. (A and B) Induction of Wip1 mRNA in the p53-positive A549 cells was monitored by northern blot analysis following: (A) γ-ray (20 Gly) or UV (30 J/m2) radiation, or (B) various stress conditions as described in Materials and methods. (C and D) Absence of Wip1 mRNA induction in the p53-deficient H1299 cells following (C) UV (30 J/m2) radiation or (D) various stress conditions as indicated. Ethidium bromide (EtBr)-stained gels of the same samples are shown at the bottom to indicate the amounts of RNA.
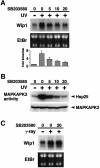
Fig. 2. Effect of the p38-specific inhibitor SB203580 on Wip1 mRNA induction. (A) Inhibition of Wip1 mRNA induction by SB203580. The inhibitor was added to A549 cells at the concentrations indicated (0, 5, 10 or 20 µM) 1 h before exposure to 30 J/m2 UV radiation, and total RNA was prepared 12 h later. The fold induction of Wip1 mRNA was determined by densitometry from three independent experiments and is indicated below each lane. Error bars indicate standard error of the mean. (B) Inhibition of UV-induced MAPKAP-K2 activity by SB203580. A549 cells were treated with SB203580 1 h before irradiation with UV (30 J/m2), and extracts were prepared 1 h later. Endogenous MAPKAP-K2 was immunoprecipitated, and its kinase activity was assessed in an in vitro kinase assay using Hsp25 as a substrate. (C) SB203580 has little effect on γ radiation-induced Wip1 expression. A549 cells were exposed to γ-ray (20 Gly) in the presence or absence of SB203580, and total RNA was prepared 4 h later.
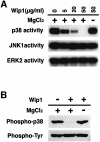
Fig. 3. Selective inhibition of the p38 MAPK pathway by Wip1 in vivo. (A) Wip1 selectively inhibits the p38 MAPK cascade. COS-7 cells were co-transfected with 0.1 µg of expression plasmids encoding Flag–p38, HA–JNK1 or HA–ERK2, with increasing amounts of the pWip1 expression vector as indicated. The total amount of transfected DNA was kept constant by including appropriate amounts of the empty vector pcDNA3. Transfected cells were serum starved for 12 h and stimulated either with UV (60 J/m2 followed by a 1 h incubation at 37°C) or with PMA (100 nM for 20 min). The activities of the epitope-tagged MAPKs were measured in immunocomplex kinase assays using specific exogenous substrates (GST–ATF2, GST–jun or MBP). The expression levels of the MAPKs in cell lysates were monitored by immunoblotting. These experiments were repeated at least three times with similar results. (B) Wip1 inhibits the activation of the p38 pathway caused by distinct stimuli. COS-7 cells were transfected with 0.3 µg/plate of either pWip1 (+) or the empty vector pcDNA3 (–), together with Flag–p38 or HA–JNK1 expression plasmid (0.1 µg). After 24 h, the cells were treated with MMS (100 µg/ml for 3 h), anisomycin (10 µg/ml for 30 min), sorbitol (0.4 M for 20 min) or left untreated (–). The activities and expression levels of Flag–p38 and HA–JNK1 were measured as described in (A). (C) Kinetics of p38 activation in response to UV radiation. A549 cells were treated with UV (30 J/m2) and cell extracts were prepared at various points in time as indicated. The phosphorylation status of endogenous p38 was analyzed by immunoblotting using antibodies specific to phosphorylated p38 (upper panel). The levels of p38 in cell lysates were indicated (lower panel).
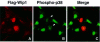
Fig. 4. Wip1 inhibits nuclear accumulation of activated p38 kinase. HeLa cells were transfected with 0.4 µg of an expression plasmid encoding Flag–Wip1. One hour after treatment with UV (60 J/m2), the cells were fixed and stained as follows: (A) Flag–Wip1 was detected with the anti-Flag M2 antibody (red). Some cells are unstained because they do not express the Flag–Wip1 protein. (B) The activated endogenous p38 MAPK was detected by the anti-phospho-specific p38 antisera (green). Cells transfected with Wip1 are indicated by arrows. (C) Flag–Wip1 and activated p38 MAPK are shown simultaneously.
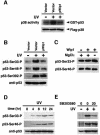
Fig. 5. Direct inactivation of the p38 MAPK by Wip1 in vivo. (A) Wip1 does not inhibit any MAPKK tested in vivo. COS-7 cells were transfected with 0.1 µg/plate of expression plasmids encoding GST–MKK6, GST–SEK1 or GST–MEK1, together with the indicated amounts of pWip1. After 18 h, the cells were serum starved for an additional 12 h and then stimulated with UV or PMA. The phosphorylation status of the affinity-purified GST–MAPKKs was analyzed by immunoblotting using antibodies specific to phosphorylated MKK6, SEK1 or MEK1. The expression level of each GST–MAPKK was also monitored using an anti-GST antibody. (B) Wip1 inhibits p38 activation induced by a constitutively active MKK6 mutant in vivo. COS-7 cells were co-transfected with Flag–p38 (0.1 µg/plate), MKK6DD (0.07 µg/plate) and Wip1 (0.2 or 0.4 µg/plate) expression plasmids as indicated. After 24 h, the kinase activity and the expression level of Flag–p38 were determined as described in Figure 2. The fold activation of kinase activity was determined by densitometrical quantitation and is indicated below each lane. (C) Co-immunoprecipitation of p38 and Wip1 expressed in vivo. COS-7 cells were transfected with 0.2 µg/plate of an expression plasmid encoding HA–p38, together with 0.2 µg of an expression plasmid encoding Flag-tagged Wip1 or vector control (pcDNAI-Flag). Cells were harvested before (–) or 1 h after (+) treatment with UV radiation. Flag–Wip1 was immunoprecipitated, and the presence of HA–p38 in the precipitates was detected by anti-HA antibody 12CA5 immunoblotting (top panel). The levels of HA–p38 (middle panel) and Flag–Wip1 (lower panel) in the total lysates are shown.
Fig. 6. In vitro dephosphorylation of the p38 MAPK by Wip1. (A) p38 can serve as a direct substrate of Wip1. Activated MAPKs were isolated from UV- or PMA-treated COS-7 cells and incubated with the indicated concentrations of purified GST–Wip1 in the presence or absence of Mg2+. Residual kinase activities were assessed in an in vitro kinase assay using specific substrates (GST–ATF2 for p38, GST–jun for JNK1, MBP for ERK2). (B) Threonine-specific dephosphorylation of p38 by Wip1 in vitro. Phosphorylated GST–p38 was affinity-purified from UV-treated COS-7 cells and incubated with purified GST–Wip1 in the presence or absence of Mg2+. The phosphorylation state of GST–p38 was examined by immunoblotting with either anti-phospho-p38 antibodies (upper panel) or anti-phosphotyrosine antibody 4G10 (lower panel).
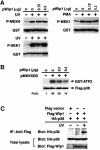
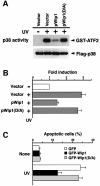
Fig. 7. Wip1 inhibits phosphorylation of p53 induced by UV radiation. (A) In vitro phosphorylation of p53 by p38. COS-7 cells were cotransfected with 0.1 µg of the expression plasmid encoding Flag–p38, with 0.3 µg of the empty vector or the pWip1 expression vector. Transfected cells were stimulated with UV, and kinase activity of Flag–p38 was assayed in an immunocomplex kinase assay as in Figure 3A, except that GST–p53 was used as a substrate. (B) Wip1 blocks phosphorylation of p53 at Ser33 and Ser46 induced by UV radiation. The p53-deficient H1299 cells were transiently transfected with 0.1 µg of an expression vector encoding wild-type p53, together with 0.3 µg of either the pWip1 expression vector or the empty vector pcDNA3 (Vector). After 18 h, cells were either treated with UV (25 J/m2) (+) or left untreated (–). Seven hours after UV treatment, cell extracts were prepared and analyzed for p53 phosphorylation by immunoblot analysis using anti-p53 antibodies specific for phosphorylation at Ser33, Ser46 or Ser392 as indicated. The levels of p53 expression in the samples were monitored using a p53-specific mAb, DO-1. (C) Wip1 does not dephosphorylate p53 at Ser33 and Ser46 in vitro. Phosphorylated p53 was immunopurified from UV-treated H1299 cells transfected with wild-type p53 expression vector. Following incubation with purified GST–Wip1, in the presence or absence of Mg2+, the phosphorylation state of p53 was examined by immunoblotting using antibodies specific for the phosphorylated Ser33 or Ser46 sites of p53. (D) Time-course of p53 phosphorylation at Ser33 and Ser46 in A549 cells. Phosphorylation status of p53 was determined in cell extracts from A549 cells treated with UV (30 J/m2) by immunoblotting using antibodies specific for p53 Ser33 (upper panel) and Ser46 (middle panel) phosphorylation sites, respectively. The levels of p53 expression in the samples were indicated (lower panel). (E) Effect of SB203580 on p53 phosphorylation at Ser33 and Ser46 in response to UV radiation. p38-specific inhibitor SB203580 were added to A549 cells 1 h before irradiation with UV (30 J/m2), and extracts were prepared 12 h later. Endogenous wt-p53 was analyzed for phosphorylation by immunoblotting as in (D). The amount of p53 in cell lysates was indicated (lower panel).
Fig. 8. Wip1 prevents p53-mediated transcription and apoptotic cell death after exposure to UV radiation. (A) p38 activation is inhibited by the wild-type Wip1 phosphatase, but not by the catalytically inactive Wip1(D/A) mutant. COS-7 cells were co-transfected with 0.1 µg/plate of the Flag–p38 plasmid and 0.3 µg of an expression plasmid encoding Wip1 or Wip1(D/A), or their vector control pcDNA3 (Vector). After 24 h, the transfected cells were treated with UV (60 J/m2), and p38 activity was assayed as in Figure 3. The level of Flag–p38 in the total extracts is shown in the lower panel. (B) Suppression of p53-mediated transcription by Wip1. MCF7 cells, which express intact p53, were transiently co-transfected with a p53–luciferase reporter plasmid pGL3-p53RE (0.1 µg) and pCMV β-galactosidase expression vector (0.07 µg), with 0.3 µg of either the control pcDNA3 (Vector), the pWip1 expression vector or the pWip1(D/A) expression vector. After 18 h, the cells were treated with UV (25 J/m2) (+) or left untreated (–). Seven hours after irradiation, luciferase activity was determined. The data represents the average fold-induction of luciferase activity from three independent experiments, in which each assay was performed in duplicate. Error bars indicate standard errors of the mean. (C) Wip1 prevents p53-mediated apoptotic cell death induced by exposure to UV radiation. A549 cells were transfected with either the pGFP expression vector, pGFP-Wip1 or pGFP-Wip1(D/A). Eighteen hours after transfection, the transfected cells were treated with UV (15 J/m2) or left untreated (None). Sixteen hours after UV treatment, the cells were fixed and their nuclei were stained with DAPI. The fraction of apoptotic cells was determined by dividing the number of GFP-positive cells that exhibit apoptotic morphology (round cell shape with condensed nucleus) by the total number of GFP-positive cells. At least 500 GFP-positive cells from five randomly chosen fields were counted. The averages of three independent experiments are shown.
Similar articles
- Osmotic shock induces G1 arrest through p53 phosphorylation at Ser33 by activated p38MAPK without phosphorylation at Ser15 and Ser20.
Kishi H, Nakagawa K, Matsumoto M, Suga M, Ando M, Taya Y, Yamaizumi M. Kishi H, et al. J Biol Chem. 2001 Oct 19;276(42):39115-22. doi: 10.1074/jbc.M105134200. Epub 2001 Aug 8. J Biol Chem. 2001. PMID: 11495913 - Phosphorylation of human p53 by p38 kinase coordinates N-terminal phosphorylation and apoptosis in response to UV radiation.
Bulavin DV, Saito S, Hollander MC, Sakaguchi K, Anderson CW, Appella E, Fornace AJ Jr. Bulavin DV, et al. EMBO J. 1999 Dec 1;18(23):6845-54. doi: 10.1093/emboj/18.23.6845. EMBO J. 1999. PMID: 10581258 Free PMC article. - Loss of Wip1 sensitizes cells to stress- and DNA damage-induced apoptosis.
Xia Y, Ongusaha P, Lee SW, Liou YC. Xia Y, et al. J Biol Chem. 2009 Jun 26;284(26):17428-37. doi: 10.1074/jbc.M109.007823. Epub 2009 Apr 24. J Biol Chem. 2009. PMID: 19395378 Free PMC article. - Wip1 phosphatase: between p53 and MAPK kinases pathways.
Goloudina AR, Kochetkova EY, Pospelova TV, Demidov ON. Goloudina AR, et al. Oncotarget. 2016 May 24;7(21):31563-71. doi: 10.18632/oncotarget.7325. Oncotarget. 2016. PMID: 26883196 Free PMC article. Review. - Wip1-dependent signaling pathways in health and diseases.
Zhu YH, Bulavin DV. Zhu YH, et al. Prog Mol Biol Transl Sci. 2012;106:307-25. doi: 10.1016/B978-0-12-396456-4.00001-8. Prog Mol Biol Transl Sci. 2012. PMID: 22340722 Review.
Cited by
- Dual-Action Kinase Inhibitors Influence p38α MAP Kinase Dephosphorylation.
Stadnicki EJ, Ludewig H, Kumar RP, Wang X, Qiao Y, Kern D, Bradshaw N. Stadnicki EJ, et al. bioRxiv [Preprint]. 2024 Aug 8:2024.05.15.594272. doi: 10.1101/2024.05.15.594272. bioRxiv. 2024. PMID: 39149408 Free PMC article. Preprint. - SOD1 is a synthetic-lethal target in _PPM1D_-mutant leukemia cells.
Zhang L, Hsu JI, Braekeleer ED, Chen CW, Patel TD, Martell AG, Guzman AG, Wohlan K, Waldvogel SM, Uryu H, Tovy A, Callen E, Murdaugh RL, Richard R, Jansen S, Vissers L, de Vries BBA, Nussenzweig A, Huang S, Coarfa C, Anastas J, Takahashi K, Vassiliou G, Goodell MA. Zhang L, et al. Elife. 2024 Jun 18;12:RP91611. doi: 10.7554/eLife.91611. Elife. 2024. PMID: 38896450 Free PMC article. - The ribotoxic stress response drives UV-mediated cell death.
Sinha NK, McKenney C, Yeow ZY, Li JJ, Nam KH, Yaron-Barir TM, Johnson JL, Huntsman EM, Cantley LC, Ordureau A, Regot S, Green R. Sinha NK, et al. Cell. 2024 Jul 11;187(14):3652-3670.e40. doi: 10.1016/j.cell.2024.05.018. Epub 2024 Jun 5. Cell. 2024. PMID: 38843833 - Unconventional activation of PRKDC by TNF-α: deciphering its crucial role in Th1-mediated inflammation beyond DNA repair as part of the DNA-PK complex.
Ghonim MA, Ju J, Pyakurel K, Ibba SV, Abouzeid MM, Rady HF, Matsuyama S, Del Valle L, Boulares AH. Ghonim MA, et al. J Inflamm (Lond). 2024 Apr 30;21(1):14. doi: 10.1186/s12950-024-00386-x. J Inflamm (Lond). 2024. PMID: 38689261 Free PMC article. - A dynamic Boolean network reveals that the BMI1 and MALAT1 axis is associated with drug resistance by limiting miR-145-5p in non-small cell lung cancer.
Gupta S, Silveira DA, Piedade GPS, Ostrowski MP, Mombach JCM, Hashimoto RF. Gupta S, et al. Noncoding RNA Res. 2023 Oct 19;9(1):185-193. doi: 10.1016/j.ncrna.2023.10.008. eCollection 2024 Mar. Noncoding RNA Res. 2023. PMID: 38125755 Free PMC article.
References
- Alessi D.R., Gómez,N., Moorhead,G., Lewis,T., Keyse,S.M. and Cohen,P. (1995) Inactivation of p42 MAP kinase by protein phosphatase 2A and a protein tyrosine phosphatase, but not CL100 in various cell lines. Curr. Biol., 5, 283–295. - PubMed
- Anderson N.G., Maller,J.L, Tonks,N.K. and Sturgill,T.W. (1990) Requirement for integration of signals from two distinct phosphorylation pathways for activation of MAP kinase. Nature, 343, 651–653. - PubMed
- Caspari T. (2000) How to activate p53. Curr. Biol., 10, R315–R317. - PubMed
- Chen Y.-R., Wang,X., Templeton,D., Davis,R.J. and Tan,T.-H. (1996) The role of c-Jun N-terminal kinase (JNK) in apoptosis induced by ultraviolet C and γ radiation: duration of JNK activation may determine cell death and proliferation. J. Biol. Chem., 271, 31929–31936. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous