A conserved clathrin assembly motif essential for synaptic vesicle endocytosis - PubMed (original) (raw)
A conserved clathrin assembly motif essential for synaptic vesicle endocytosis
J R Morgan et al. J Neurosci. 2000.
Abstract
Although clathrin assembly by adaptor proteins (APs) plays a major role in the recycling of synaptic vesicles, the molecular mechanism that allows APs to assemble clathrin is poorly understood. Here we demonstrate that AP180, like AP-2 and AP-3, binds to the N-terminal domain of clathrin. Sequence analysis reveals a motif, containing the sequence DLL, that exists in multiple copies in many clathrin APs. Progressive deletion of these motifs caused a gradual reduction in the ability of AP180 to assemble clathrin in vitro. Peptides from AP180 or AP-2 containing this motif also competitively inhibited clathrin assembly by either protein. Microinjection of these peptides into squid giant presynaptic terminals reversibly blocked synaptic transmission and inhibited synaptic vesicle endocytosis by preventing coated pit formation at the plasma membrane. These results indicate that the DLL motif confers clathrin assembly properties to AP180 and AP-2 and, perhaps, to other APs. We propose that APs promote clathrin assembly by cross-linking clathrin triskelia via multivalent interactions between repeated DLL motifs in the APs and complementary binding sites on the N-terminal domain of clathrin. These results reveal the structural basis for clathrin assembly and provide novel insights into the molecular mechanism of clathrin-mediated synaptic vesicle endocytosis.
Figures
Fig. 1.
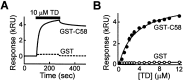
Binding of clathrin TD to AP180. A, Time course of binding of TD to AP180 was monitored by SPR. Traces illustrate the SPR response, expressed in kilo response units (kRU). During the time indicated by the_bar_, TD was passed over surfaces to which either GST-C58 (solid line) or GST (dashed line) had been coupled covalently. B, TD binds to GST-C58 in a dose-dependent manner, whereas TD does not bind to GST at all of the concentrations that were tested. Different concentrations of TD were passed over the surfaces, and the responses were followed over time. Maximum response values are plotted as a function of TD concentration. The data points represent the mean response in two experiments. Error bars indicate ± SEM. The data were fit by a rectangular hyperbolic function.
Fig. 2.
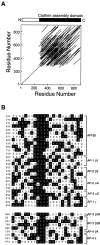
Identification of a repetitive element in the APs.A, A PLALIGN self-homology plot of AP180 reveals that the structure of the clathrin assembly domain of AP180 (residues 305–901) is highly repetitive. The parallel lines indicate regions of similarity. B, Inspection of the LALIGN alignments revealed that the repetitive element is an ∼23 amino acid degenerate sequence, typically included on a core DLL motif. The AP180 repeats were aligned with similar repeats found in the large subunits of the AP-1 and AP-2 adaptors by Megalign. Below, Alignment of the large subunits of the AP-3 and AP-4 adaptors revealed a related repetitive element that typically included a core SLL motif. The residues surrounded by_black_ in the alignments are identical to a structural similarity consensus (see Materials and Methods).
Fig. 3.
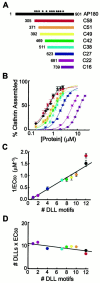
Deletion of DLL motifs reduces the ability of AP180 to assemble clathrin. A, Recombinant deletion mutants of AP180 were constructed to vary the number of DLL motifs in the clathrin assembly domain (C58). The DLL motifs within full-length AP180 are denoted by asterisks.B, Deletion of the DLL motifs gradually reduced the ability of the AP180 mutants to assemble clathrin in vitro. Colors correspond to the constructs indicated in A for B–D; the data points represent the mean of three to six independent experiments; error bars indicate ± SEM. Smooth lines indicate the Hill equation fit to the data.C, The clathrin assembly activity of the AP180 mutants was a linear function of the number of DLL motifs. The concentration of assembly protein to give half-maximal assembly (EC 50) was determined from fitting the data in B to the Hill equation. The error bars indicate ± SEM. D, Clathrin assembly is proportional to the concentration of DLL motifs, because the product of the EC50 × the number of DLLs motifs was constant for each construct. The EC50 values were determined from the data in B.
Fig. 4.
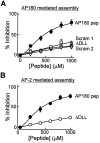
A DLL-containing peptide from the clathrin assembly domain of AP180 (AP180 pep) inhibits clathrin assembly in vitro by both AP180 and AP-2.A, AP180 pep produced a concentration-dependent inhibition of clathrin assembly by AP180. The inhibition by AP180 pep was reduced when the DLL was mutated to AAA (Δ DLL) or when the sequence of amino acids within the peptide was scrambled (Scram1 and_Scram2_). B, Concentration-dependent inhibition of AP-2-mediated clathrin assembly by AP180 pep. The inhibition by AP180 pep was reduced when the DLL was mutated to AAA (Δ DLL). In both panels the data points represent the mean of three to four independent experiments; error bars indicate ± SEM. Smooth lines are fits of the Hill equation.
Fig. 5.
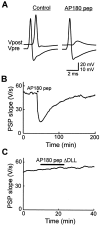
AP180 pep inhibits neurotransmitter release.A, Recordings of presynaptic and postsynaptic responses before (Control) and after the injection of AP180 pep. AP180 pep transiently reduced transmitter release below the threshold for eliciting a postsynaptic action potential. The vertical scale bar applies to Vpost (above) and_Vpre_ (below) traces. B, Time course of the inhibition of transmitter release by AP180 pep, (injected during the time indicated by the bar), as measured by the slope of the postsynaptic potential (PSP).C, AP180 pep ΔDLL had no effect on transmitter release.
Fig. 6.
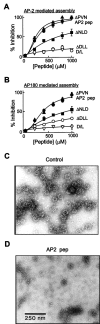
A DLL-containing AP-2 peptide (AP2 pep) inhibits clathrin assembly in vitro.A, B, Clathrin assembly by both AP-2 (A) and AP180 (B) was inhibited by AP2 pep in a concentration-dependent manner, whereas a peptide in which the DLL was mutated to AAA (Δ DLL) or in which the D and L residues were exchanged (D/L) had little effect. Mutation of the downstream PVN to AAA in AP2 pep (Δ PVN) did not reduce the efficacy of the peptide, whereas the mutation of the adjacent NLD to AAA (Δ NLD) had a moderate effect.Data points represent the mean of three to four independent experiments; error bars indicate ± SEM. Smooth lines are fits of the Hill equation. C, D, The ability of AP2 pep to inhibit clathrin assembly also was examined by negative-staining electron microscopy of clathrin cages that were assembled in the presence of AP2 pep ΔDLL (Control) or AP2 peptide (AP2 pep). The scale bar in D applies to both images.
Fig. 7.
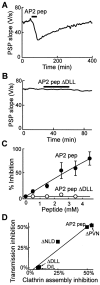
AP2 pep also inhibits transmitter release.A, AP2 pep reversibly inhibited transmitter release when it was injected (bar) into the squid giant presynaptic terminal. B, The inhibitory effect on transmission was lost when the DLL was mutated (AP2 pep Δ DLL).C, Inhibition of transmitter release by AP2 pep was dependent on peptide concentration. _Data points_represent the mean inhibition measured in two to seven experiments; error bars represent ± SEM. Smooth lines indicate rectangular hyperbola fits to the data. D, Correlation between the abilities of several AP-2 peptides to inhibit synaptic transmission in vivo and clathrin assembly in vitro by AP-2.
Fig. 8.
Presynaptic terminals injected with AP2 pep are depleted of synaptic vesicles. Terminals injected with AP2 pep had fewer synaptic vesicles than those injected with AP2 pep ΔDLL (Control). The postsynaptic spines are indicated by S; the scale bar applies to both images.
Fig. 9.
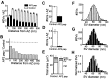
The DLL-containing AP2 pep inhibits synaptic vesicle endocytosis at the clathrin assembly step. A, Spatial distribution of synaptic vesicles (SVs) in terminals injected with AP2 pep or controls. Data represent the mean values and SEM from 209 active zones (AZs) analyzed from two AP2 peptide-injected terminals and 184 AZs from two control terminals. B, Relative spatial distribution of SVs, determined by dividing the mean values for the AP2 pep by the control shown in A. The dashed line indicates a 1:1 ratio and represents no difference between terminals injected with AP2 pep or control peptide. C, D, AP2 pep significantly reduced the mean number of SVs (C) and coated vesicles (CVs; D) found per AZ.E, The mean area of membrane within vesicular endosomes (Endo), SVs, CVs, and the presynaptic plasma membrane (PM) was measured for each section in control and AP2 peptide-injected terminals. F–H, Distribution of SV diameters was measured from 20 active zones each in control (F) or AP2 peptide-injected terminals (G). The smooth lines indicate Gaussian functions fit to these distributions. H, Superimposition of the two distributions (normalized to the peak) reveals no change in mean SV diameter after injection of the AP2 peptide.
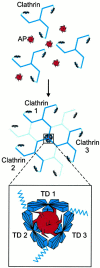
Fig. 10.
Cross-linking model for clathrin assembly by APs. Multiple clathrin binding elements (shown as spikes) exist within a single clathrin AP (top). These clathrin binding elements allow a single AP to have multivalent interactions with TDs from several clathrin molecules, thereby assembling them into a cage (middle). The inset(bottom) shows our proposed arrangement of TDs from three clathrin molecules at a vertex and illustrates the multivalent cross-linking of these clathrin molecules by APs.
Similar articles
- A role for the clathrin assembly domain of AP180 in synaptic vesicle endocytosis.
Morgan JR, Zhao X, Womack M, Prasad K, Augustine GJ, Lafer EM. Morgan JR, et al. J Neurosci. 1999 Dec 1;19(23):10201-12. doi: 10.1523/JNEUROSCI.19-23-10201.1999. J Neurosci. 1999. PMID: 10575017 Free PMC article. - Eps15 homology domain-NPF motif interactions regulate clathrin coat assembly during synaptic vesicle recycling.
Morgan JR, Prasad K, Jin S, Augustine GJ, Lafer EM. Morgan JR, et al. J Biol Chem. 2003 Aug 29;278(35):33583-92. doi: 10.1074/jbc.M304346200. Epub 2003 Jun 14. J Biol Chem. 2003. PMID: 12807910 - Dual interaction of synaptotagmin with mu2- and alpha-adaptin facilitates clathrin-coated pit nucleation.
Haucke V, Wenk MR, Chapman ER, Farsad K, De Camilli P. Haucke V, et al. EMBO J. 2000 Nov 15;19(22):6011-9. doi: 10.1093/emboj/19.22.6011. EMBO J. 2000. PMID: 11080148 Free PMC article. - Endocytosis: an assembly protein for clathrin cages.
McMahon HT. McMahon HT. Curr Biol. 1999 May 6;9(9):R332-5. doi: 10.1016/s0960-9822(99)80206-1. Curr Biol. 1999. PMID: 10330371 Review. - Clathrin and synaptic vesicle endocytosis: studies at the squid giant synapse.
Augustine GJ, Morgan JR, Villalba-Galea CA, Jin S, Prasad K, Lafer EM. Augustine GJ, et al. Biochem Soc Trans. 2006 Feb;34(Pt 1):68-72. doi: 10.1042/BST0340068. Biochem Soc Trans. 2006. PMID: 16417485 Free PMC article. Review.
Cited by
- Accumulation of α-synuclein triggered by presynaptic dysfunction.
Nakata Y, Yasuda T, Fukaya M, Yamamori S, Itakura M, Nihira T, Hayakawa H, Kawanami A, Kataoka M, Nagai M, Sakagami H, Takahashi M, Mizuno Y, Mochizuki H. Nakata Y, et al. J Neurosci. 2012 Nov 28;32(48):17186-96. doi: 10.1523/JNEUROSCI.2220-12.2012. J Neurosci. 2012. PMID: 23197711 Free PMC article. - The PICALM protein plays a key role in iron homeostasis and cell proliferation.
Scotland PB, Heath JL, Conway AE, Porter NB, Armstrong MB, Walker JA, Klebig ML, Lavau CP, Wechsler DS. Scotland PB, et al. PLoS One. 2012;7(8):e44252. doi: 10.1371/journal.pone.0044252. Epub 2012 Aug 30. PLoS One. 2012. PMID: 22952941 Free PMC article. - A role for an Hsp70 nucleotide exchange factor in the regulation of synaptic vesicle endocytosis.
Morgan JR, Jiang J, Oliphint PA, Jin S, Gimenez LE, Busch DJ, Foldes AE, Zhuo Y, Sousa R, Lafer EM. Morgan JR, et al. J Neurosci. 2013 May 1;33(18):8009-21. doi: 10.1523/JNEUROSCI.4505-12.2013. J Neurosci. 2013. PMID: 23637191 Free PMC article. - Synergy between intrinsically disordered domains and structured proteins amplifies membrane curvature sensing.
Zeno WF, Baul U, Snead WT, DeGroot ACM, Wang L, Lafer EM, Thirumalai D, Stachowiak JC. Zeno WF, et al. Nat Commun. 2018 Oct 8;9(1):4152. doi: 10.1038/s41467-018-06532-3. Nat Commun. 2018. PMID: 30297718 Free PMC article. - Weak Molecular Interactions in Clathrin-Mediated Endocytosis.
Smith SM, Baker M, Halebian M, Smith CJ. Smith SM, et al. Front Mol Biosci. 2017 Nov 14;4:72. doi: 10.3389/fmolb.2017.00072. eCollection 2017. Front Mol Biosci. 2017. PMID: 29184887 Free PMC article. Review.
References
- Cowles CR, Odorizzi G, Payne GS, Emr SD. The AP-3 adaptor complex is essential for cargo-selective transport to the yeast vacuole. Cell. 1997;91:109–118. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 NS021624/NS/NINDS NIH HHS/United States
- NS29051/NS/NINDS NIH HHS/United States
- NS21624/NS/NINDS NIH HHS/United States
- R56 NS029051/NS/NINDS NIH HHS/United States
- R01 NS029051/NS/NINDS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous