Activation of Valpha14(+) natural killer T cells by alpha-galactosylceramide results in development of Th1 response and local host resistance in mice infected with Cryptococcus neoformans - PubMed (original) (raw)
Activation of Valpha14(+) natural killer T cells by alpha-galactosylceramide results in development of Th1 response and local host resistance in mice infected with Cryptococcus neoformans
K Kawakami et al. Infect Immun. 2001 Jan.
Abstract
We examined the effect of alpha-galactosylceramide (alpha-GalCer) on the synthesis of gamma interferon (IFN-gamma) and local resistance in mice infected intravenously with Cryptococcus neoformans. The level of IFN-gamma in serum increased on day 3, reached a peak level on day 7, and decreased to the basal level on day 14 postinfection in mice treated with alpha-GalCer, while in vehicle-treated mice, no increase was detected at any time points except for a small increase on day 7. Such effects were not observed in NKT-KO mice. In CD4KO mice, minor synthesis of IFN-gamma was detected on day 3 in sera but was completely abolished by day 7. The alpha-GalCer-induced IFN-gamma production on day 3 was partially reduced in mice depleted of NK cells by treatment with anti-asialo-GM(1) antibody (Ab). Spleen cells obtained from infected and alpha-GalCer-treated mice on day 7 produced a large amount of IFN-gamma upon restimulation with live organisms, while only a marginal level of production was detected in splenocytes from infected and vehicle-treated mice. Such effects were abolished in CD4KO and NKT-KO mice. Finally, the fungal loads in the lungs and spleen on days 7 and 14 were significantly reduced in alpha-GalCer-treated mice compared to those in control mice. In NKT-KO mice, local resistance elicited by alpha-GalCer was completely abolished, although no obvious exacerbation of infection was detected. Furthermore, treatment with anti-IFN-gamma monoclonal Ab mostly abrogated the protective effect of this agent. Thus, our results indicated that activation of Valpha14(+) NKT cells resulted in an increased Th1 response and local resistance to C. neoformans through production of IFN-gamma.
Figures
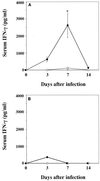
FIG. 1
α-GalCer treatment increases the IFN-γ level in serum. Mice received intraperitoneal injections of α-GalCer (2 μg/mouse) or the same volume of vehicle on days 0, 3, and 7 after intravenous injection of C. neoformans (106/mouse) (A) or the same volume of normal saline (B). On days 0, 3, 7, and 14, the mice were sacrificed and the levels of IFN-γ in serum were measured. Each symbol represents the mean and SD for three mice. Experiments were repeated three times with similar results. Open circles, vehicle; solid circles, α-GalCer. ∗, P < 0.05 compared with vehicle-treated mice.
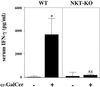
FIG. 2
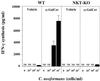
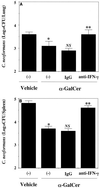
α-GalCer treatment causes NKT-cell-dependent induction of IFN-γ. WT and NKT-KO mice received intraperitoneal injections of α-GalCer (2 μg/mouse) or the same volume of vehicle on days 0 and 3 after intravenous injection of C. neoformans (106/mouse). On day 7, the mice were sacrificed and the levels of IFN-γ in serum were measured. Each bar represents the mean and SD for three mice. Experiments were repeated twice with similar results. NS, not significant; ∗, P < 0.05 compared with vehicle-treated mice.
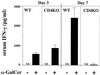
FIG. 3
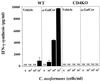
Effect of α-GalCer treatment on the IFN-γ in serum in CD4KO mice. WT and CD4KO mice received intraperitoneal injections of α-GalCer (2 μg/mouse) or the same volume of vehicle on days 0 and 3 after intravenous injection of C. neoformans (106/mouse). On days 3 and 7, the mice were sacrificed and the levels of IFN-γ in serum were measured. Each bar represents the mean and SD for three mice. Experiments were repeated four times with similar results. ND, not detected.
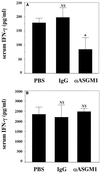
FIG. 4
Effect of NK-cell depletion on a α-GalCer-induced IFN-γ production. WT mice received intraperitoneal injections of α-GalCer (2 μg/mouse) on days 0 and 3 after intravenous injection of C. neoformans (106/mouse). These mice were injected intraperitoneally with phosphate-buffered saline (PBS), rabbit IgG, or anti-ASGM1 Ab on days −3, 0, and 3 after infection. On days 3 (A) and 7 (B), the mice were sacrificed and the levels of IFN-γ in serum were measured. Each bar represents the mean and SD for three mice. Experiments were repeated twice with similar results. ND, not detected. NS, not significant; ∗, P < 0.05 compared with PBS-treated mice.
FIG. 5
Induction of Th1 cells by α-GalCer treatment. WT and CD4KO mice received intraperitoneal injections of α-GalCer (2 μg/mouse) or the same volume of vehicle on days 0 and 3 after intravenous injection of C. neoformans (106/mouse). On day 7, spleen cells were prepared and restimulated with the indicated doses of live fungal organisms for 2 days. Then the concentration of IFN-γ in the culture supernatants was measured by ELISA. Each bar represents the mean and SD for triplicate cultures. Experiments were repeated three times with similar results. ND, not detected.
FIG. 6
NKT-cell-dependent induction of Th1 cells by α-GalCer treatment. WT and NKT-KO mice received intraperitoneal injections of α-GalCer (2 μg/mouse) or the same volume of vehicle on days 0 and 3 after intravenous injection of C. neoformans (106/mouse). On day 7, spleen cells were prepared and restimulated with the indicated doses of live fungal organisms for 2 days. Then the concentration of IFN-γ in the culture supernatants was measured by ELISA. Each bar represents the mean and SD for triplicate cultures. Experiments were repeated twice with similar results. ND, not detected.
FIG. 7
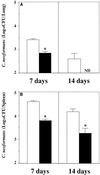
Effect of α-GalCer treatment on local host resistance to cryptococcal infection. WT mice received intraperitoneal injections of α-GalCer (2 μg/mouse) or the same volume of vehicle on days 0, 3, and 7 after intravenous injection of C. neoformans (106/mouse). On days 7 and 14, the live colonies in the lungs (A) and spleen (B) were counted. Each bar represents the mean and SD for three mice. Experiments were repeated three times with similar results. Open bars, vehicle-treated mice; solid bars, α-GalCer-treated mice; ND, not detected; ∗, P < 0.05 compared with the vehicle-treated mice.
FIG. 8
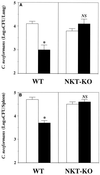
α-GalCer causes NKT-cell-dependent host resistance to cryptococcal infection. WT and NKT-KO mice were treated with intraperitoneal injections of α-GalCer (2 μg/mouse) or the same volume of vehicle on days 0 and 3 after intravenous injection of C. neoformans (106/mouse). On day 7, the live colonies in the lungs (A) and spleen (B) were counted. Each bar represents the mean and SD for six mice. NS, not significant; ∗, P < 0.05 compared with vehicle-treated mice.
FIG. 9
Effect of anti-IFN-γ MAb on α-GalCer-induced host resistance to cryptococcal infection. WT mice were intraperitoneally injected with α-GalCer (2 μg/mouse) or the same volume of vehicle on days 0 and 3 after intravenous injection of C. neoformans (106/mouse). The α-GalCer-treated mice were injected intraperitoneally with 200 μg of anti-IFN-γ MAb or control rat IgG on days −1, 0, and 3 after infection. On day 7, the live colonies in the lungs (A) and spleen (B) were counted. Each bar represents the mean and SD for six mice. ∗, P < 0.05 compared with vehicle-treated mice; NS, not significant; ∗∗, P < 0.05 compared with α-GalCer-treated and Ab-untreated mice.
Similar articles
- Enhanced gamma interferon production through activation of Valpha14(+) natural killer T cells by alpha-galactosylceramide in interleukin-18-deficient mice with systemic cryptococcosis.
Kawakami K, Kinjo Y, Yara S, Uezu K, Koguchi Y, Tohyama M, Azuma M, Takeda K, Akira S, Saito A. Kawakami K, et al. Infect Immun. 2001 Nov;69(11):6643-50. doi: 10.1128/IAI.69.11.6643-6650.2001. Infect Immun. 2001. PMID: 11598033 Free PMC article. - A novel function of Valpha14+CD4+NKT cells: stimulation of IL-12 production by antigen-presenting cells in the innate immune system.
Tomura M, Yu WG, Ahn HJ, Yamashita M, Yang YF, Ono S, Hamaoka T, Kawano T, Taniguchi M, Koezuka Y, Fujiwara H. Tomura M, et al. J Immunol. 1999 Jul 1;163(1):93-101. J Immunol. 1999. PMID: 10384104 - Critical contribution of IFN-gamma and NK cells, but not perforin-mediated cytotoxicity, to anti-metastatic effect of alpha-galactosylceramide.
Hayakawa Y, Takeda K, Yagita H, Kakuta S, Iwakura Y, Van Kaer L, Saiki I, Okumura K. Hayakawa Y, et al. Eur J Immunol. 2001 Jun;31(6):1720-7. Eur J Immunol. 2001. PMID: 11385616 - Innate Valpha14(+) natural killer T cells mature dendritic cells, leading to strong adaptive immunity.
Fujii S, Shimizu K, Hemmi H, Steinman RM. Fujii S, et al. Immunol Rev. 2007 Dec;220:183-98. doi: 10.1111/j.1600-065X.2007.00561.x. Immunol Rev. 2007. PMID: 17979847 Review. - Role of alpha-galactosylceramide-activated Valpha14 natural killer T cells in the regulation of allergic diseases.
Iwamura C, Nakayama T. Iwamura C, et al. Allergol Int. 2007 Mar;56(1):1-6. doi: 10.2332/allergolint.R-06-136. Epub 2007 Jan 29. Allergol Int. 2007. PMID: 17259803 Review.
Cited by
- The NKG2D-activating receptor mediates pulmonary clearance of Pseudomonas aeruginosa.
Borchers MT, Harris NL, Wesselkamper SC, Zhang S, Chen Y, Young L, Lau GW. Borchers MT, et al. Infect Immun. 2006 May;74(5):2578-86. doi: 10.1128/IAI.74.5.2578-2586.2006. Infect Immun. 2006. PMID: 16622193 Free PMC article. - Functions of CD1d-Restricted Invariant Natural Killer T Cells in Antimicrobial Immunity and Potential Applications for Infection Control.
Kinjo Y, Takatsuka S, Kitano N, Kawakubo S, Abe M, Ueno K, Miyazaki Y. Kinjo Y, et al. Front Immunol. 2018 Jun 6;9:1266. doi: 10.3389/fimmu.2018.01266. eCollection 2018. Front Immunol. 2018. PMID: 29928278 Free PMC article. Review. - Cross-talk between cd1d-restricted nkt cells and γδ cells in t regulatory cell response.
Liu W, Huber SA. Liu W, et al. Virol J. 2011 Jan 21;8:32. doi: 10.1186/1743-422X-8-32. Virol J. 2011. PMID: 21255407 Free PMC article. Review. - Clinical development of a novel CD1d-binding NKT cell ligand as a vaccine adjuvant.
Padte NN, Li X, Tsuji M, Vasan S. Padte NN, et al. Clin Immunol. 2011 Aug;140(2):142-51. doi: 10.1016/j.clim.2010.11.009. Epub 2010 Dec 24. Clin Immunol. 2011. PMID: 21185784 Free PMC article. Review. - Commentary: Role of Sterylglucosidase 1 (Sgl1) on the pathogenicity of Cryptococcus neoformans: potential applications for vaccine development.
Martinez LR. Martinez LR. Front Microbiol. 2015 Oct 9;6:1112. doi: 10.3389/fmicb.2015.01112. eCollection 2015. Front Microbiol. 2015. PMID: 26500643 Free PMC article. No abstract available.
References
- Carnaud C, Lee D, Donnars O, Park S-H, Beavis A, Koezuka Y, Bendelac A. Cross-talk between cells of the innate immune system: NKT cells rapidly activate NK cells. J Immunol. 1999;163:4647–4650. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials