Presenilin-mediated transmembrane cleavage is required for Notch signal transduction in Drosophila - PubMed (original) (raw)
Presenilin-mediated transmembrane cleavage is required for Notch signal transduction in Drosophila
G Struhl et al. Proc Natl Acad Sci U S A. 2001.
Abstract
The cleavage model for signal transduction by receptors of the LIN-12/Notch family posits that ligand binding leads to cleavage within the transmembrane domain, so that the intracellular domain is released to translocate to the nucleus and activate target gene expression. The familial Alzheimer's disease-associated protein Presenilin is required for LIN-12/Notch signaling, and several lines of evidence suggest that Presenilin mediates the transmembrane cleavage event that releases the LIN-12/Notch intracellular domain. However, doubt was cast on this possibility by a report that Presenilin is not required for the transducing activity of N(ECN), a constitutively active transmembrane form of Notch, in Drosophila. Here, we have reassessed this finding and show instead that Presenilin is required for activity of N(ECN) for all cell fate decisions examined. Our results indicate that transmembrane cleavage and signal transduction are strictly correlated, supporting the cleavage model for signal transduction by LIN-12/Notch and a role for Presenilin in mediating the ligand-induced transmembrane cleavage.
Figures
Figure 1
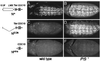
Presenilin-dependent activity of Notch in the embryonic central nervous system. Neuroblasts marked by the expression of Hb protein are shown in wild-type (A, C, and E) and _PS_− (B,D, and F) embryos that ubiquitously express either N+ (A and B), NECN (C and D), or Nintra (E and F) under Gal4/UAS control. (A) Ubiquitous expression of N+ in otherwise wild-type embryos has little or no effect on neuroblast segregation. (C and E) In contrast, ubiquitous expression of either NECN or Nintra suppresses neuroblast segregations, indicating constitutive transducing activity. In _PS_− embryos, most or all ventral ectodermal cells segregate as neuroblasts, even when N+ or NECN are ubiquitously expressed (B and D), indicating that the transducing activity of these transmembrane forms requires Presenilin. In contrast, neuroblast segregation is suppressed in_PS_− embryos expressing Nintra (F), indicating that this form of Notch bypasses the requirement for Presenilin. All embryos are shown in ventral aspect just after completion of germ-band elongation (anterior to the left). The structure of the Notch proteins are shown schematically: EGF, epidermal growth factor motifs; LNR, LIN-12/Notch repeat motifs; TM, transmembrane domain; CDC10, CDC10/SWI6 motifs.
Figure 2
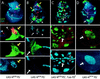
Presenilin is required for the transducing activity of transmembrane but not intracellular forms of Notch during wing development. Each column contains views of a single mosaic imaginal wing disk. Clones that lack PS activity are marked by GFP expression (green). These clones also express either NECN or Nintra, which are constitutively active in a wild-type genetic background. smc-Z expression (red) labels SMCs that will form mechanosensory bristles in the presumptive notum (n) as well as sense organs in other portions of the disk. Cut expression (blue) marks subsets of SMCs. Cut is also expressed in a thin stripe of “edge cell” straddling the dorsoventral compartment boundary (vd) in the wing blade, and in adepithelial cells associated with the notum. The disk shown in A contains only a single small clone and hence serves as a reference for the normal disk. Disks are shown anterior to the left and ventral down. (A) Series of views of a disk carrying one small clone of _PS_− _UAS_-N ECN_-expressing cells (yellow arrow). Cells within the clone form a cluster of SMCs (marked by smc-Z and Cut expression) instead of the single SMC that would normally form at this position, indicating the absence of Notch transducing activity. Single SMCs (marked by_smc-Z expression) in neighboring wild-type tissue are indicated by arrowheads. (B) This disk carries multiple_PS_− clones that express NECN and display phenotypes associated with the absence of Notch transducing activity. Two clones in the notum (yellow arrows) show the formation of SMC clusters in place of single SMCs; two clones in the wing blade span the dorsoventral compartment boundary (white arrow and arrowhead) and show a cell autonomous loss of Cut expression. (C) This disk serves as a control. Clones of _PS_− _UAS_-N _ECN_-expressing cells were generated in the presence of a rescuing_Tub_-PS+ transgene. Restoration of Presenilin activity from the rescuing transgene reveals the constitutive activity of NECN within clones. In the notum, SMCs do not form, indicating that neurogenesis is suppressed. There is no overlap between the clone (green) and smc-Z expression (red); the few apparent cases are due to superimposition of the nuclear GFP and cytoplasmic smc-Z signals in different focal planes. In the wing blade, clones autonomously express Cut, indicating constitutive activity of Notch (white arrow marks a representative clone). These clones are also associated with overgrowth of neighboring, wild-type tissue, an indication that the mutant cells are ectopically expressing Wg as well as Cut. (D) This disk carries several clones of _PS_− _UAS_-N intra_-expressing cells. Clones in the wing blade autonomously express Cut. The wing blade clone marked by the white arrow has also induced neighboring wild-type cells to express the smc-Z reporter, an indication that these cells are developing as wing margin bristles in response to Wg ectopically expressed by the mutant cells within the clone (see also Fig. 3_C).
Figure 3
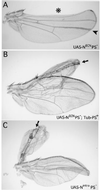
Adult wing phenotypes associated with_PS_− clones that express transmembrane or nuclear forms of Notch. Clones are marked by expression of Yellow under UAS/Gal4 control, which darkens cuticular structures, including wing hairs and margin bristles (not visible in the figure). (A) _PS_− clones that express NECN display phenotypes associated with the absence of Notch signal transduction. One clone (*) crosses the wing margin (which coincides with the dorsoventral compartment boundary) and displays extensive wing notching. A second clone (arrowhead) within the dorsal compartment of the blade forms an abnormally thick vein. (B) A clone of PS_− cells that express NECN in the presence of the rescuing_Tub_-PS+ transgene displays constitutive Notch activity (arrow). The clone is associated with a double dorsal wing outgrowth which is flanked by adjacent rows of dorsal wing margin bristles (here, as in C, an arrow points down the plane of mirror symmetry). Note that in this experiment, the presence of the clone could not be scored on the margin bristles because the Tub_-PS+ transgene used in this experiment includes a_yellow+ gene which rescues normal (dark) pigmentation in the bristles. However, the contribution of the clone to the rest of the wing blade could still be scored because this_yellow+ gene does not rescue pigmentation of the wing hairs. (C) A clone of Nintra-expressing _PS_− cells (arrow) associated with a double dorsal wing outgrowth which coincides with a thin strip of mutant cells flanked by adjacent rows of dorsal wing margin bristles formed by neighboring wild-type cells.
Similar articles
- Presenilin is required for activity and nuclear access of Notch in Drosophila.
Struhl G, Greenwald I. Struhl G, et al. Nature. 1999 Apr 8;398(6727):522-5. doi: 10.1038/19091. Nature. 1999. PMID: 10206646 - Neurogenic phenotypes and altered Notch processing in Drosophila Presenilin mutants.
Ye Y, Lukinova N, Fortini ME. Ye Y, et al. Nature. 1999 Apr 8;398(6727):525-9. doi: 10.1038/19096. Nature. 1999. PMID: 10206647 - Nicastrin is required for Presenilin-mediated transmembrane cleavage in Drosophila.
Chung HM, Struhl G. Chung HM, et al. Nat Cell Biol. 2001 Dec;3(12):1129-32. doi: 10.1038/ncb1201-1129. Nat Cell Biol. 2001. PMID: 11781576 - Notch and presenilin: a proteolytic mechanism emerges.
Fortini ME. Fortini ME. Curr Opin Cell Biol. 2001 Oct;13(5):627-34. doi: 10.1016/s0955-0674(00)00261-1. Curr Opin Cell Biol. 2001. PMID: 11544033 Review. - Notch and presenilins in vertebrates and invertebrates: implications for neuronal development and degeneration.
Selkoe DJ. Selkoe DJ. Curr Opin Neurobiol. 2000 Feb;10(1):50-7. doi: 10.1016/s0959-4388(99)00054-9. Curr Opin Neurobiol. 2000. PMID: 10679435 Review.
Cited by
- How Drosophila melanogaster Forms its Mechanoreceptors.
Furman DP, Bukharina TA. Furman DP, et al. Curr Genomics. 2008;9(5):312-23. doi: 10.2174/138920208785133271. Curr Genomics. 2008. PMID: 19471605 Free PMC article. - Su(H)-mediated repression positions gene boundaries along the dorsal-ventral axis of Drosophila embryos.
Ozdemir A, Ma L, White KP, Stathopoulos A. Ozdemir A, et al. Dev Cell. 2014 Oct 13;31(1):100-13. doi: 10.1016/j.devcel.2014.08.005. Dev Cell. 2014. PMID: 25313963 Free PMC article. - The Arp2/3 complex and WASp are required for apical trafficking of Delta into microvilli during cell fate specification of sensory organ precursors.
Rajan A, Tien AC, Haueter CM, Schulze KL, Bellen HJ. Rajan A, et al. Nat Cell Biol. 2009 Jul;11(7):815-24. doi: 10.1038/ncb1888. Epub 2009 Jun 21. Nat Cell Biol. 2009. PMID: 19543274 Free PMC article. - Notch and the awesome power of genetics.
Greenwald I. Greenwald I. Genetics. 2012 Jul;191(3):655-69. doi: 10.1534/genetics.112.141812. Genetics. 2012. PMID: 22785620 Free PMC article. Review. - Genetic regions that interact with loss- and gain-of-function phenotypes of deltex implicate novel genes in Drosophila Notch signaling.
Hori K, Fuwa TJ, Seki T, Matsuno K. Hori K, et al. Mol Genet Genomics. 2005 Feb;272(6):627-38. doi: 10.1007/s00438-004-1098-1. Epub 2005 Jan 14. Mol Genet Genomics. 2005. PMID: 15650868
References
- Greenwald I. Genes Dev. 1998;12:1751–1762. - PubMed
- Joutel A, Tournier-Lasserve E. Semin Cell Dev Biol. 1998;9:619–625. - PubMed
- Blaumueller C M, Qui H, Zagouras P, Artavanis-Tsakonas S. Cell. 1997;90:281–291. - PubMed
- Brou C, Logeat F, Gupta N, Bessia C, LeBail O, Doedens J R, Cumano A, Roux P, Black R A, Israel A. Molec Cell. 2000;5:207–216. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous