Processive DNA helicase activity of the minichromosome maintenance proteins 4, 6, and 7 complex requires forked DNA structures - PubMed (original) (raw)
Processive DNA helicase activity of the minichromosome maintenance proteins 4, 6, and 7 complex requires forked DNA structures
J K Lee et al. Proc Natl Acad Sci U S A. 2001.
Abstract
The minichromosome maintenance (Mcm) proteins 2-7 are required for both the initiation and elongation steps of chromosomal DNA replication. Previous studies have shown that the Mcm complex consisting of the Mcm 4, 6, and 7 proteins contains 3' to 5' DNA helicase activity with limited processivity (displacing duplex DNA regions up to 30 nt). In this report, we show that the presence of both 5' and 3' single-stranded tails in DNA helicase substrates is essential for the processive helicase activity of the Mcm complex. The presence of both 5' and 3' tails facilitated the formation of double heterohexameric complexes of Mcm4/6/7 on substrate DNA, which appeared to be essential for the processive helicase activity. The double heterohexameric complex of Mcm4/6/7, in the presence of a single-strand DNA binding protein, is capable of unwinding duplex DNA region of about 600 bp in length. These results support the hypothesis that the Mcm4/6/7 complex can function as a replication helicase.
Figures
Figure 1
Influence of the presence of a 5′ tail on the helicase activity of Mcm4/6/7 complex. DNA helicase activity assays were carried out with increasing amounts of Mcm4/6/7 protein and 5 fmol of the indicated substrates with (B) or without (A) a 40-nt oligo(dT) tail at its 5′ end, as described in Materials and Methods. Lane B, boiled substrate; lane 1, no Mcm4/6/7 protein was added; lanes 2–5 contained 25, 50, 100, or 200 ng of the Mcm4/6/7 complex, respectively.
Figure 2
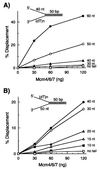
DNA substrates containing both 5′ and 3′ ss tails are essential for processive helicase activity of the Mcm4/6/7 complex. (A) Increasing levels of the Mcm4/6/7 complex were incubated with 10 fmol of helicase substrates containing a 40-nt 5′ oligo(dT) ss region and varying lengths of 3′ ss tail as indicated. □, Reactions carried out with a substrate containing no 3′ tail; ◊, substrate with a 30-nt 3′ tail; ▴, substrate containing a 40-nt 3′ tail; ○, substrate with a 50-nt 3′ tail; ●, substrate with a 60-nt 3′ tail. (B) Helicase assays were performed with 10 fmol of DNA containing a 3′ oligo(dT) ss region of 50 nt in length and varying lengths of oligo(dT) at the 5′ end as indicated. □, No 5′ tail; ■, 10-nt 5′ tail; ◊, 15-nt 5′ tail; ▴, 20-nt 5′ tail; ○, 30-nt 5′ tail; and ●, 40-nt 5′ tail.
Figure 3
Binding of the Mcm complex to various DNA helicase substrate. Gel mobility shift assays were performed with three different DNA substrates (each at 10 fmol) containing both a 60-nt 3′ tail and a 30-nt 5′ tail (A), a 60-nt 3′ tail alone (B), or a 30-nt 5′ tail alone (C). Lane 1, no Mcm4/6/7 protein was added; lanes 2–6, reactions were carried out with 2.2, 6.7, 20, 60, and 180 ng of the Mcm4/6/7 complex, respectively. C1 and C2 represent DNA–Mcm protein complexes.
Figure 4
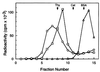
Glycerol gradient sedimentation analysis of Mcm/DNA complexes. Mcm4/6/7 complex was incubated with 0.5 pmol of a32P-labeled DNA substrate containing a 60-nt 3′ tail and a 30-nt 5′ tail with a 50-bp duplex region (the same substrate described in Fig. 3_A_) in a reaction mixture (150 μl) containing 25 mM Hepes-NaOH, pH 7.5, 50 mM sodium acetate, 10 mM magnesium acetate, 1 mM DTT, and 0.1 mg/ml BSA. After incubation at 25°C for 30 min, the reaction mixture was loaded onto a 5-ml 15–35% glycerol gradient in a buffer A containing 0.1 mg/ml BSA. After centrifugation at 48,000 rpm for 6 h in a Beckman SW 50.1 rotor at 4°C, fractions (330 μl) were collected from the bottom of the tube. The distribution of Mcm/DNA complexes or DNA were determined by liquid scintillation counting. The marker proteins used were thyroglobulin (Thy, 19S), catalase (Cat, 11.3S), and BSA (BSA, 4.3S). ○, The binding reaction was carried out with 7.5 μg of the Mcm4/6/7 complex (13 pmol Mcm4/6/7 dimer) to assemble the C2 complex; □, 0.3 μg of the Mcm4/6/7 complex was used (about 0.5 pmol Mcm4/6/7 dimer) for the formation of the C1 complex; ◊, DNA substrate alone.
Figure 5
Crosslinking of the Mcm4/6/7 complex bound to helicase DNA substrates. The Mcm4/6/7 complex (2 μg) was incubated with 0.2 pmol of the biotinylated DNA containing both a 60-nt 3′ tail and a 30-nt 5′ tail or a substrate containing a 3′ tail alone (the same substrates as described in Fig. 3 A and_B_) under identical conditions used in the gel mobility shift assay. After binding of the Mcm/DNA complex to streptavidin-coated magnetic beads, the beads were washed twice with buffer A. Crosslinking was carried out at three different concentrations of BS3 (0.05, 0.1, or 0.2 mM, respectively) as described in Materials and Methods. Crosslinked proteins were electrophoresed through SDS/4.5% PAGE and then stained with silver. The arrowhead indicates the position of the dimeric complex of Mcm4/6/7, and the asterisk indicates the position of the double hexameric Mcm4/6/7 complex.
Figure 6
Processivity of the Mcm4/6/7 helicase. DNA helicase activity was assayed by using a partial duplex M13 substrate (5 fmol) containing duplex regions varying in length between 40 and 600 bp and a 40-nt oligo(dT) tail on its 5′ end. Indicated amounts of the Mcm4/6/7 complex were preincubated with DNA substrates in the helicase reaction buffer at 25°C for 10 min, and E. coli SSB was added as indicated. After incubation at 32°C for 1 h, reactions were stopped by the addition of 4 μl 5× reaction stop buffer (100 mM EDTA/0.5% SDS) and proteinase K (2 μg), and reaction mixtures were incubated for an additional 30 min at 37°C. Aliquots then were electrophoresed through a 2.5% low melting agarose gel in 1× TBE at 120 V for 3 h. Lane M, 32P-labeled 50-bp ladder DNA marker (denatured); lane 1, boiled substrate; lanes 2 and 12, reactions without Mcm4/6/7 protein; + and + + denote the addition of 200 and 400 ng of E. coli SSB, respectively.
Similar articles
- Cdt1 forms a complex with the minichromosome maintenance protein (MCM) and activates its helicase activity.
You Z, Masai H. You Z, et al. J Biol Chem. 2008 Sep 5;283(36):24469-77. doi: 10.1074/jbc.M803212200. Epub 2008 Jul 7. J Biol Chem. 2008. PMID: 18606811 Free PMC article. - Biochemical analysis of the intrinsic Mcm4-Mcm6-mcm7 DNA helicase activity.
You Z, Komamura Y, Ishimi Y. You Z, et al. Mol Cell Biol. 1999 Dec;19(12):8003-15. doi: 10.1128/MCB.19.12.8003. Mol Cell Biol. 1999. PMID: 10567526 Free PMC article. - Identification and characterization of a novel component of the human minichromosome maintenance complex.
Sakwe AM, Nguyen T, Athanasopoulos V, Shire K, Frappier L. Sakwe AM, et al. Mol Cell Biol. 2007 Apr;27(8):3044-55. doi: 10.1128/MCB.02384-06. Epub 2007 Feb 12. Mol Cell Biol. 2007. PMID: 17296731 Free PMC article. - The MCM helicase: linking checkpoints to the replication fork.
Forsburg SL. Forsburg SL. Biochem Soc Trans. 2008 Feb;36(Pt 1):114-9. doi: 10.1042/BST0360114. Biochem Soc Trans. 2008. PMID: 18208397 Review. - Initiating DNA synthesis: from recruiting to activating the MCM complex.
Lei M, Tye BK. Lei M, et al. J Cell Sci. 2001 Apr;114(Pt 8):1447-54. doi: 10.1242/jcs.114.8.1447. J Cell Sci. 2001. PMID: 11282021 Review.
Cited by
- Thymine-rich single-stranded DNA activates Mcm4/6/7 helicase on Y-fork and bubble-like substrates.
You Z, Ishimi Y, Mizuno T, Sugasawa K, Hanaoka F, Masai H. You Z, et al. EMBO J. 2003 Nov 17;22(22):6148-60. doi: 10.1093/emboj/cdg576. EMBO J. 2003. PMID: 14609960 Free PMC article. - RACK1 promotes lung cancer cell growth via an MCM7/RACK1/ Akt signaling complex.
Fei L, Ma Y, Zhang M, Liu X, Luo Y, Wang C, Zhang H, Zhang W, Han Y. Fei L, et al. Oncotarget. 2017 Jun 20;8(25):40501-40513. doi: 10.18632/oncotarget.17120. Oncotarget. 2017. PMID: 28465488 Free PMC article. - Schizosaccharomyces pombe pfh1+ encodes an essential 5' to 3' DNA helicase that is a member of the PIF1 subfamily of DNA helicases.
Zhou JQ, Qi H, Schulz VP, Mateyak MK, Monson EK, Zakian VA. Zhou JQ, et al. Mol Biol Cell. 2002 Jun;13(6):2180-91. doi: 10.1091/mbc.02-02-0021. Mol Biol Cell. 2002. PMID: 12058079 Free PMC article. - Essential role of phosphorylation of MCM2 by Cdc7/Dbf4 in the initiation of DNA replication in mammalian cells.
Tsuji T, Ficarro SB, Jiang W. Tsuji T, et al. Mol Biol Cell. 2006 Oct;17(10):4459-72. doi: 10.1091/mbc.e06-03-0241. Epub 2006 Aug 9. Mol Biol Cell. 2006. PMID: 16899510 Free PMC article. - DNA helicase activity is associated with the replication initiator protein rep of tomato yellow leaf curl geminivirus.
Clérot D, Bernardi F. Clérot D, et al. J Virol. 2006 Nov;80(22):11322-30. doi: 10.1128/JVI.00924-06. Epub 2006 Aug 30. J Virol. 2006. PMID: 16943286 Free PMC article.
References
- Kearsey S E, Labib K. Biochim Biophys Acta. 1998;1398:113–136. - PubMed
- Tye B K. Annu Rev Biochem. 1999;68:649–686. - PubMed
- Aparicio O M, Weinstein D M, Bell S P. Cell. 1997;91:59–69. - PubMed
- Tanaka T, Knapp D, Nasmyth K. Cell. 1997;90:649–660. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials