Cells adapted to the proteasome inhibitor 4-hydroxy- 5-iodo-3-nitrophenylacetyl-Leu-Leu-leucinal-vinyl sulfone require enzymatically active proteasomes for continued survival - PubMed (original) (raw)
Comparative Study
. 2001 Jan 16;98(2):513-8.
doi: 10.1073/pnas.98.2.513. Epub 2001 Jan 9.
Affiliations
- PMID: 11149939
- PMCID: PMC14618
- DOI: 10.1073/pnas.98.2.513
Comparative Study
Cells adapted to the proteasome inhibitor 4-hydroxy- 5-iodo-3-nitrophenylacetyl-Leu-Leu-leucinal-vinyl sulfone require enzymatically active proteasomes for continued survival
M F Princiotta et al. Proc Natl Acad Sci U S A. 2001.
Abstract
The proteasome is the primary protease used by cells for degrading proteins and generating peptide ligands for class I molecules of the major histocompatibility complex. Based on the properties of cells adapted to grow in the presence of the proteasome inhibitor 4-hydroxy-5-iodo-3-nitrophenylacetyl-Leu-Leu-leucinal-vinyl sulfone (NLVS), it was proposed that proteasomes can be replaced by alternative proteolytic systems, particularly a large proteolytic complex with a tripeptidyl peptidase II activity. Here we show that NLVS-adapted cells retain sensitivity to a number of highly specific proteasome inhibitors with regard to antigenic peptide generation, accumulation of polyubiquitinated proteins, degradation of p53, and cell viability. In addition, we show that in the same assays (with a single minor exception), NLVS-adapted cells are about as sensitive as nonselected cells to Ala-Ala-Phe-chloromethylketone, a specific inhibitor of tripeptidyl peptidase II activity. Based on these findings, we conclude that proteasomes still have essential proteolytic functions in adapted cells that are not replaced by Ala-Ala-Phe-chloromethylketone-sensitive proteases.
Figures
Figure 1
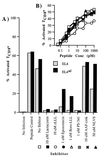
Effect of proteasome inhibitors on antigen processing in EL4 and EL4ad cells. Cells were treated with proteasome inhibitors and then infected with PR8 influenza virus. TCD8+ cells specific for influenza NP366–374 were added 1 h after infection. Brefeldin A was added 5 h after infection. After an additional 4 h, cells were harvested, stained for CD8 and intracellular IFN-γ, and analyzed by cytofluorography. (A) The percentage of CD8+ cells positive for intracellular IFN-γ is represented graphically. (B) To ensure that proteasome inhibitors were not inhibiting IFN-γ production in TCD8+ cells, the assay described in A was performed with the use of EL4 cells titrated with limiting quantities of NP366–374 peptide as antigen-presenting cells and TCD8+ cells exposed to the same inhibitor concentrations as in A. Legend symbols are defined in A.
Figure 2
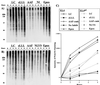
Effect of proteasome inhibitors on the accumulation of polyUb proteins in EL4 and EL4ad cells. (A and_B_) EL4 (A) and EL4ad (B) cells treated with inhibitors for 2, 4, and 8 h were analyzed by Western blotting with the use of the FK2 mAb, the binding of which was visualized by chemiluminescence. The inhibitors used were 10 μM lactacystin (LC), 10 μM zLLL (zLLL), 10 μM AAF-cmk (AAF), no inhibitor (NI), 50 μM NLVS (NLVS), and 1 μM epoxomicin (Epox). (C) The increase in FK2 staining was quantitated and is represented graphically.
Figure 3
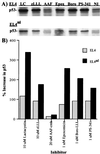
Effect of proteasome inhibitors on the accumulation of p53 in EL4 and EL4ad cells. Aliquots from cells analyzed in Fig. 2 were Western blotted with the use of polyclonal p53-specific antibodies. The inhibitors used were 10 μM lactacystin (LC), 10 μM zLLL (zLLL), 10 μM AAF-cmk (AAF), 1 μM epoxomicin (Epox), 1 μM boro-LLL (Boro), 1 μM PS-341 (PS-341), and no inhibitor (NI). (A) Antibody binding was visualized by chemiluminescence. (B) The increase in p53 staining was quantitated and is represented graphically.
Figure 4
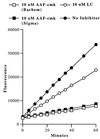
Effect of AAF-cmk on AAF-amc hydrolysis in EL4ad cells. EL4ad cells were treated with 10 μM AAF-cmk or 10 μM lactacystin for 90 min at 37°C and then incubated with 100 μM AAF-amc. Hydrolysis of AAF-amc was determined by measuring fluorescence at 5–10-min intervals.
Similar articles
- Protease inhibitor-induced apoptosis: accumulation of wt p53, p21WAF1/CIP1, and induction of apoptosis are independent markers of proteasome inhibition.
An WG, Hwang SG, Trepel JB, Blagosklonny MV. An WG, et al. Leukemia. 2000 Jul;14(7):1276-83. doi: 10.1038/sj.leu.2401812. Leukemia. 2000. PMID: 10914553 - Accumulation of polyubiquitylated proteins in response to Ala-Ala-Phe-chloromethylketone is independent of the inhibition of Tripeptidyl peptidase II.
Villasevil EM, Guil S, López-Ferreras L, Sánchez C, Del Val M, Antón LC. Villasevil EM, et al. Biochim Biophys Acta. 2010 Sep;1803(9):1094-105. doi: 10.1016/j.bbamcr.2010.06.001. Epub 2010 Jun 8. Biochim Biophys Acta. 2010. PMID: 20553980 - Proteasome inhibitors: valuable new tools for cell biologists.
Lee DH, Goldberg AL. Lee DH, et al. Trends Cell Biol. 1998 Oct;8(10):397-403. doi: 10.1016/s0962-8924(98)01346-4. Trends Cell Biol. 1998. PMID: 9789328 Review. - Generation of major histocompatibility complex class I antigens: functional interplay between proteasomes and TPPII.
Kloetzel PM. Kloetzel PM. Nat Immunol. 2004 Jul;5(7):661-9. doi: 10.1038/ni1090. Nat Immunol. 2004. PMID: 15224091 Review.
Cited by
- TDP-43 redistribution is an early event in sporadic amyotrophic lateral sclerosis.
Giordana MT, Piccinini M, Grifoni S, De Marco G, Vercellino M, Magistrello M, Pellerino A, Buccinnà B, Lupino E, Rinaudo MT. Giordana MT, et al. Brain Pathol. 2010 Mar;20(2):351-60. doi: 10.1111/j.1750-3639.2009.00284.x. Epub 2009 Mar 17. Brain Pathol. 2010. PMID: 19338576 Free PMC article. - Primed for Interactions: Investigating the Primed Substrate Channel of the Proteasome for Improved Molecular Engagement.
Loy CA, Trader DJ. Loy CA, et al. Molecules. 2024 Jul 17;29(14):3356. doi: 10.3390/molecules29143356. Molecules. 2024. PMID: 39064934 Free PMC article. Review. - A marked reduction in priming of cytotoxic CD8+ T cells mediated by stress-induced glucocorticoids involves multiple deficiencies in cross-presentation by dendritic cells.
Hunzeker JT, Elftman MD, Mellinger JC, Princiotta MF, Bonneau RH, Truckenmiller ME, Norbury CC. Hunzeker JT, et al. J Immunol. 2011 Jan 1;186(1):183-94. doi: 10.4049/jimmunol.1001737. Epub 2010 Nov 22. J Immunol. 2011. PMID: 21098225 Free PMC article. - Insulin-like growth factor-I mediates neuroprotection in proteasome inhibition-induced cytotoxicity in SH-SY5Y cells.
Cheng B, Maffi SK, Martinez AA, Acosta YP, Morales LD, Roberts JL. Cheng B, et al. Mol Cell Neurosci. 2011 Jul;47(3):181-90. doi: 10.1016/j.mcn.2011.04.002. Epub 2011 Apr 23. Mol Cell Neurosci. 2011. PMID: 21545837 Free PMC article. - Varied Role of Ubiquitylation in Generating MHC Class I Peptide Ligands.
Wei J, Zanker D, Di Carluccio AR, Smelkinson MG, Takeda K, Seedhom MO, Dersh D, Gibbs JS, Yang N, Jadhav A, Chen W, Yewdell JW. Wei J, et al. J Immunol. 2017 May 15;198(10):3835-3845. doi: 10.4049/jimmunol.1602122. Epub 2017 Mar 31. J Immunol. 2017. PMID: 28363906 Free PMC article.
References
- Baumeister W, Walz J, Zuhl F, Seemuller E. Cell. 1998;92:367–380. - PubMed
- DeMartino G N, Slaughter C A. J Biol Chem. 1999;274:22123–22126. - PubMed
- Deveraux Q, Ustrell V, Pickart C, Rechsteiner M. J Biol Chem. 1994;269:7059–7061. - PubMed
- Yewdell J W, Antón L C, Bennink J R. J Immunol. 1996;157:1823–1826. - PubMed
- Rock K L, Goldberg A L. Annu Rev Immunol. 1999;17:739–779. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous