Glycocalyx on rabbit intestinal M cells displays carbohydrate epitopes from Muc2 - PubMed (original) (raw)
Glycocalyx on rabbit intestinal M cells displays carbohydrate epitopes from Muc2
H Lelouard et al. Infect Immun. 2001 Feb.
Abstract
It is essential to investigate the apical surface properties of both M cells and dome enterocytes to understand the mechanisms involved in the binding of pathogens to M cells. In rabbit appendix tissue, monoclonal antibodies (MAbs) highlight differences between M cells (MAb 58) and dome enterocytes (MAb 214). Such antibodies ultimately recognized intestinal mucin-related epitopes. To further characterize these differences, the labeling patterns obtained with these MAbs were compared to those obtained with other antibodies to intestinal mucins on dissected domes from all gut-associated lymphoid tissues. A glycoprotein recognized by MAb 58 was purified on a CsCl isopycnic density gradient and microsequenced, and its mRNA expression was localized by in situ hybridization. It was identified as the rabbit homologue of human Muc2, i.e., the major mucin secreted in intestine tissue. Two other Muc2 carbohydrate epitopes were also expressed on M cells, although Muc2 mRNA was not detected. All results indicated that M cells express, on their apical membrane, glycoconjugates bearing at least three glycosidic epitopes from Muc2. MAb 214 and MAb 6G2, which recognized a partially characterized mucin expressed on dome enterocytes, were negative markers for M cells in rabbit gut-associated lymphoid tissues. We propose that the presence, on the surface of M cells, of carbohydrates also expressed on Muc2, together with the absence of an enterocyte-associated mucin, could favor pathogen attachment and accessibility to the M-cell luminal membrane.
Figures
FIG. 1
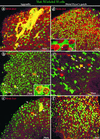
Complementary labeling of M cells and enterocytes by MAb 58 and MAb 214 in rabbit appendix and Peyer's patch FAE. Whole domes from a rabbit appendix (a, b) or a Peyer's patch (c, d) were fixed, double labeled with dichlorotriazinyl aminofluorescein (DTAF)-coupled MAb 58 (a, c) and with MAb 214 and secondary RITC-coupled antibody (b, d), and viewed by confocal microscopy, as described in Materials and Methods. Panels a and b and panels c and d, respectively, are surface views of the same two fields. Note complementarity between M-cell (a, c) and enterocyte (b, d) apical surface staining in both tissues (see arrows pointing to M cells [a and b] and to enterocytes [c and d]). M cells appear to be surrounded by enterocytes in the appendix (a, b) whereas they appear to encompass enterocytes in Peyer's patches (c, d).
FIG. 2
Double labeling of whole domes of rabbit appendix and distal Peyer's patches with MAb 58 and other antibodies to mucin-related epitopes. Direct labeling with DTAF-coupled MAb 58 (green) in the appendix (a through c) and in distal Peyer's patches (d through f) was concentrated on the apex of M cells. (a) Indirect labeling of the appendix with MAb 6G2-RITC (red) was located on the apices of dome enterocytes. Filamentous networks of adherent mucus extending over several adjacent cells appeared yellow in color due to colocalization of MAb 58 and MAb 6G2 (arrowheads). (b) Indirect labeling of appendix tissue with MAb 5H7-RITC (red) was observed on a few round-shaped goblet cells at the base of domes (arrows), where it partially colocalized with MAb 58. Note the higher-magnification inset showing the double-labeled secretory granules of the two delineated adjacent goblet cells. (c) Indirect labeling of appendix with MAb 3A4-RITC (red) was present on rare dome enterocytes (arrows) and on adherent mucus (arrowheads). (d) Labeling of a distal Peyer's patch with MAb 214-RITC (red) together with MAb 58 (green) displays a typical mosaic pattern. At higher magnification (inset), packed microvilli are clearly recognizable. A circular area of one M cell appeared not to be labeled by MAb 58 (arrow). (e) Indirect labeling with MAb 5H7-RITC (red) was present on less than 10% of M cells, which are recognizable by their star shape. Colocalization with MAb 58 on these M cells appeared in various colors from green to red (arrows). (f) Indirect labeling with MAb 3A4-RITC (red) was observed on M cells which appeared in various shades of red, yellow, and green due to different concentrations of the two carbohydrate epitopes recognized by MAb 58 and MAb 3A4.
FIG. 3
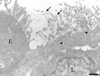
Electron micrograph of an M cell labeled with MAb 3A4. The labeling (10-nm gold) was concentrated on the microvilli (arrows) and on the membranes of apical vesicles from M cells (arrowheads). Enterocytes (E) and lymphocytes in the basolateral pocket (L) were devoid of significant labeling. Bar, 2 μm.
FIG. 4
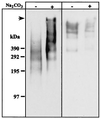
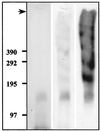
Antigen 58 enrichment and antigen 214 partial solubilization in a modified preparation of carbonate-washed membranes. Immunoblotting with MAb 58 (left panel) and MAb 214 (right panel) of 50 μg of total membranes treated with Na2CO3, or untreated, run on an SDS–2 to 10% PAGE gradient under reducing (for MAb 58) or nonreducing (for MAb 214) conditions, followed by transfer to an Immobilon-P membrane. The top of the gel (arrow) and molecular masses (in kilodaltons) are indicated on the left-hand side of the figure.
FIG. 5
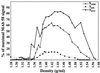
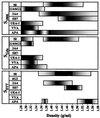
Antigen 58 distribution after different extraction procedures and on a CsCl isopycnic density gradient. The initial membrane preparation (100,000 × g pellet) was carbonate washed and submitted successively to 6 M gdn-HCl, 0.5% Triton X-100 and 10 mM DTT, as described in Materials and Methods. The 100,000 × g (S100K, ⧫), gdn-HCl (Sgdn, ■) and DTT (SDTT, ▴) supernatants were subjected to density gradient centrifugation in 6 M CsCl–gdn-HCl. Fractions of 1.1 ml were collected from the top to the bottom of the gradient and analyzed for density, and 2 μl of each fraction was used for a dot blot assay of reactivity with MAb 58. No signal was detected for the Scarb and STX fractions.
FIG. 6
Distribution of mucin-related epitopes and lectin-detected sugars after CsCl isopycnic density gradient centrifugation. The fractions collected from CsCl isopycnic density gradients of S100K, Sgdn, and SDTT and containing antigen 58 (see Fig. 5) were further analyzed for dot blot reactivity with other mucin-related MAbs and lectins. Reactivities that were >20% (grey) and >90% (black) of the maximal signal obtained with the corresponding MAbs or lectins are indicated as a function of the density.
FIG. 7
Biochemical characterization of purified antigen 58 from Sgdn. An aliquot of the Sgdn fraction containing antigen 58 (see Fig. 6) was run on an SDS-2 to 10% PAGE gradient under reducing conditions, transferred to Immobilon-P membranes, stained with Coomassie blue (first lane) and then PAS (second lane), and immunolabeled with MAb 58 (third lane). The top of the gel (arrow) and molecular sizes (in kilodaltons) are indicated on the left-hand side of the figure.
FIG. 8
Immunoprecipitation of SDTT fractions. The SDTT fractions (see Fig. 6) containing either antigens 58 and 3A4 (left panel) or antigens 58 and 5H7 (right panel) were divided into 30-μl aliquots. These aliquots were run on an SDS-2 to 10% PAGE gradient without (control) or after immunoprecipitation (IP) with MAb 58 (IP 58) or MAb 5H7 (IP 5H7) coupled to protein A-Sepharose. After transfer to Immobilon-P membrane, Western blotting was performed with MAb 58, MAb 3A4, or MAb 5H7. The top of the gel (arrow) and molecular sizes (in kilodaltons) are indicated on the left. Immunoprecipitation with MAb 3A4 was not performed since it was an IgM molecule and could not be used the same way.
FIG. 9
: Biochemical characterization of antigen 214- and 6G2-enriched fractions from S100K. Thirty microliters of the antigen 214- or 6G2-enriched fraction from S100K (see Fig. 6) was treated as indicated above the lanes, run on SDS-2 to 10% gradient PAGE, and transferred to Immobilon-P membrane before Coomassie blue staining and immunolabeling with either MAb 214 or MAb 6G2. The top of the gel (arrow) and molecular sizes (in kilodaltons) are indicated at the left.
FIG. 10
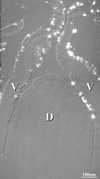
In situ hybridization of Muc2 probe to rabbit distal Peyer's patch. Superposition of differential interference contrast and fluorescein-Tyramide signal amplification of the Muc2 probe showed the absence of staining of the dome epithelium (D), whereas goblet cells on the adjacent villi (V) were intensely stained.
Similar articles
- Mucin-related epitopes distinguish M cells and enterocytes in rabbit appendix and Peyer's patches.
Lelouard H, Reggio H, Mangeat P, Neutra M, Montcourrier P. Lelouard H, et al. Infect Immun. 1999 Jan;67(1):357-67. doi: 10.1128/IAI.67.1.357-367.1999. Infect Immun. 1999. PMID: 9864237 Free PMC article. - Heterogeneity of mucin gene expression in normal and neoplastic tissues.
Ho SB, Niehans GA, Lyftogt C, Yan PS, Cherwitz DL, Gum ET, Dahiya R, Kim YS. Ho SB, et al. Cancer Res. 1993 Feb 1;53(3):641-51. Cancer Res. 1993. PMID: 7678777 - The mucus and mucins of the goblet cells and enterocytes provide the first defense line of the gastrointestinal tract and interact with the immune system.
Pelaseyed T, Bergström JH, Gustafsson JK, Ermund A, Birchenough GM, Schütte A, van der Post S, Svensson F, Rodríguez-Piñeiro AM, Nyström EE, Wising C, Johansson ME, Hansson GC. Pelaseyed T, et al. Immunol Rev. 2014 Jul;260(1):8-20. doi: 10.1111/imr.12182. Immunol Rev. 2014. PMID: 24942678 Free PMC article. Review. - Mucin gene expression in the rat middle ear: an improved method for RNA harvest.
Lin J, Ho S, Shekels L, Paparella MM, Kim Y. Lin J, et al. Ann Otol Rhinol Laryngol. 1999 Aug;108(8):762-8. doi: 10.1177/000348949910800809. Ann Otol Rhinol Laryngol. 1999. PMID: 10453784 Review.
Cited by
- Mucin dynamics and enteric pathogens.
McGuckin MA, Lindén SK, Sutton P, Florin TH. McGuckin MA, et al. Nat Rev Microbiol. 2011 Apr;9(4):265-78. doi: 10.1038/nrmicro2538. Nat Rev Microbiol. 2011. PMID: 21407243 Review. - Intestinal M cells: the fallible sentinels?
Miller H, Zhang J, Kuolee R, Patel GB, Chen W. Miller H, et al. World J Gastroenterol. 2007 Mar 14;13(10):1477-86. doi: 10.3748/wjg.v13.i10.1477. World J Gastroenterol. 2007. PMID: 17461437 Free PMC article. Review. - MUC1 cell surface mucin is a critical element of the mucosal barrier to infection.
McAuley JL, Linden SK, Png CW, King RM, Pennington HL, Gendler SJ, Florin TH, Hill GR, Korolik V, McGuckin MA. McAuley JL, et al. J Clin Invest. 2007 Aug;117(8):2313-24. doi: 10.1172/JCI26705. J Clin Invest. 2007. PMID: 17641781 Free PMC article. - The Peyer's Patch Mononuclear Phagocyte System at Steady State and during Infection.
Da Silva C, Wagner C, Bonnardel J, Gorvel JP, Lelouard H. Da Silva C, et al. Front Immunol. 2017 Oct 2;8:1254. doi: 10.3389/fimmu.2017.01254. eCollection 2017. Front Immunol. 2017. PMID: 29038658 Free PMC article. Review. - Mucins in the mucosal barrier to infection.
Linden SK, Sutton P, Karlsson NG, Korolik V, McGuckin MA. Linden SK, et al. Mucosal Immunol. 2008 May;1(3):183-97. doi: 10.1038/mi.2008.5. Epub 2008 Mar 5. Mucosal Immunol. 2008. PMID: 19079178 Free PMC article. Review.
References
- Carlstedt I, Herrmann A, Karlsson H, Sheehan J, Fransson L A, Hansson G C. Characterization of two different glycosylated domains from the insoluble mucin complex of rat small intestine. J Biol Chem. 1993;268:18771–18781. - PubMed
- Chambraud L, Bernadac A, Gorvel J P, Maroux S. Renewal of goblet cell mucus granules during the cell migration along the crypt-villus axis in rabbit jejunum: an immunolabeling study. Biol Cell. 1989;65:151–162. - PubMed
- Clark M A, Jepson M A, Simmons N L, Booth T A, Hirst B H. Differential expression of lectin-binding sites defines mouse intestinal M-cells. J Histochem Cytochem. 1993;41:1679–1687. - PubMed
- Clark M A, Jepson M A, Simmons N L, Hirst B H. Differential surface characteristics of M cells from mouse intestinal Peyer's and caecal patches. Histochem J. 1994;26:271–280. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous