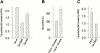Activation of the COOH-terminal Src kinase (Csk) by cAMP-dependent protein kinase inhibits signaling through the T cell receptor - PubMed (original) (raw)
Activation of the COOH-terminal Src kinase (Csk) by cAMP-dependent protein kinase inhibits signaling through the T cell receptor
T Vang et al. J Exp Med. 2001.
Abstract
In T cells, cAMP-dependent protein kinase (PKA) type I colocalizes with the T cell receptor-CD3 complex (TCR/CD3) and inhibits T cell function via a previously unknown proximal target. Here we examine the mechanism for this PKA-mediated immunomodulation. cAMP treatment of Jurkat and normal T cells reduces Lck-mediated tyrosine phosphorylation of the TCR/CD3 zeta chain after T cell activation, and decreases Lck activity. Phosphorylation of residue Y505 in Lck by COOH-terminal Src kinase (Csk), which negatively regulates Lck, is essential for the inhibitory effect of cAMP on zeta chain phosphorylation. PKA phosphorylates Csk at S364 in vitro and in vivo leading to a two- to fourfold increase in Csk activity that is necessary for cAMP-mediated inhibition of TCR-induced interleukin 2 secretion. Both PKA type I and Csk are targeted to lipid rafts where proximal T cell activation occurs, and phosphorylation of raft-associated Lck by Csk is increased in cells treated with forskolin. We propose a mechanism whereby PKA through activation of Csk intersects signaling by Src kinases and inhibits T cell activation.
Figures
Figure 1
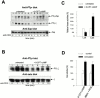
cAMP inhibits TCR/CD3-induced ζ chain phosphorylation. (A) The phosphotyrosine content of Zap-70 and ζ chain were examined (top and middle, respectively) in anti–Zap-70 immunoprecipitates from untreated (lanes 1–6) and 8-CPT-cAMP–pretreated (300 μM for 30 min; lanes 7–12) Jurkat cells stimulated with anti-CD3 Ab (OKT-3) for the indicated time (0–15 min). Anti–Zap-70 immunoblotting verified equal amounts of Zap-70 (bottom). (B) ζ chain phosphorylation (top) was examined in peripheral T cell lysates after anti-CD3 Ab stimulation (0–10 min) of untreated (lanes 1–4) and 8-CPT-cAMP–pretreated (500 μM for 15 min; lanes 5–8) cells. Anti–ζ chain immunoblotting verified equal loading (bottom). (C) Tyrosine kinase activities of Lck and Csk were assessed in immunoprecipitates of Jurkat T cells either treated with 8-CPT-cAMP (300 μM) for 20 min (black bars) or untreated (white bars; means ± SEM). Immunoblotting verified comparable amounts of Lck and Csk in immunoprecipitates from 8-CPT-cAMP–treated and untreated cells. (D) Csk activities in untreated peripheral T cells (white bars) and cells treated with 8-CPT-cAMP (500 μM for 5 min) or IBMX (200 μg/ml for 15 min) together with PGE2 (100 μM for 5 min; black bars) were assessed as in C. In parallel experiments, PGE2 treatment produced cAMP levels that increased from 1.6 ± 0.4 to 20.8 ± 7.3 pmol/106 cells. For the data presented in A and B, one representative of three experiments is shown.
Figure 2
cAMP-mediated inhibition of TCR/CD3-induced ζ chain phosphorylation is dependent on inactivation of Lck by phosphorylation at Y505. Lck-deficient JCaM1 cells were transfected with empty pEF vector alone (vector), with wild-type (wt) Lck, or with mutant Lck-Y505F, where the COOH-terminal Y505-regulatory site is mutated to resist inactivation by Csk, and incubated (30 min) in the absence or presence of 8-CPT-cAMP (300 μM) followed by incubation in the absence or presence of anti-CD3 Ab (5 min). PO3-ζ chains (top) were fished with GST-Zap-70-(SH2)2 and detected by phosphotyrosine immunoblotting (reference 40). All lanes contain the endogenous truncated Lck present in JCaM1 cells (catalytically inactive; lower bands), whereas full-length Lck is present in equal amounts in transfected cells (lower panel; lanes 5–12). One representative of three experiments is shown.
Figure 3
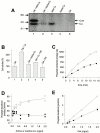
PKA-mediated phosphorylation increases the tyrosine kinase activity of Csk. (A) Csk (10 ng/μl) was incubated with native Cα (5 ng/μl active; lane 1) and heat-inactivated (65°C for 10 min) Cα (lane 2) and [γ-32P]ATP and subjected to SDS-PAGE and autoradiography. Native Cα alone (lane 3), heat-inactivated Cα alone (lane 4), and Csk incubated alone (lane 5) were included as controls. Arrows indicate phosphorylated Csk (50 kD) and autophosphorylated Cα (40 kD). (B) Csk (1 ng/μl) kinase activity when incubated alone 1, in the presence of native 2, or heat-inactivated (65°C for 10 min) 3 Cα (2 ng/μl; means ± SD, n = 5). Coincubation of Csk and PKI (85 μM) with 4 or without 5 native Cα is also shown. (C) Time-dependent phosphorylation of pEY by Csk (1 ng/μl) in the presence of native (○) and heat-inactivated (65°C for 10 min) (•) Cα (2 ng/μl). Each experiment was performed with single point measurements, and one representative of a total of seven assays is shown. (D) The effects of different amounts (0–2 ng/μl) of native (○) and heat-inactivated (•) Cα on Csk (1 ng/μl)-catalyzed phosphate transfer to pEY. Duplicate measurements were performed, and one representative assay of a total of four is shown. (E) Csk (0–2 ng/μl) concentration-dependent phosphotransfer in the presence of a constant amount of native (2 ng/μl active; ○) or heat-inactivated (•) Cα. All samples were assayed in duplicate and error bars (half range) are shown. Where error bars are not visible, they are within the point. One representative experiment of four is presented.
Figure 4
PKA phosphorylation increases the tyrosine kinase activity of Csk towards an endogenous substrate. Tyrosine phosphorylation of heat-inactivated (65°C for 10 min) purified Lck enzyme (30 ng/μl, cat. no. 14-106; Upstate Biotechnology) by Csk (0.3 ng/μl) was assessed either in the presence or absence of PKA catalytic subunit Cα (10 ng/μl) in a buffer containing 5 mM Mg2+ and 200 μM ATP at 30°C for 10 min. Reactions were stopped by the addition of SDS sample buffer, subjected to SDS-PAGE, and phosphotyrosine content of Lck was assessed by antiphosphotyrosine immunoblotting (4G10). Densitometric scanning was performed to evaluate the level of Csk-mediated tyrosine phosphorylation of Lck in the absence and presence of PKA. n.d., not done.
Figure 6
PKA phosphorylation of Csk-S364 is necessary for the regulatory effect of cAMP on Csk. (A) In vitro Csk kinase activities of Csk-wt, Csk-S364C, and Csk-S339A/S340A/T341A (Csk-AAA) were examined in the presence of active or heat-inactivated PKA Cα. (B) Csk kinase activity in anti-HA immunoprecipitates from Jurkat TAg T cells cotransfected with HA-Csk-wt and PKA Cβ subunit inserted in pEFneo in sense or reverse orientation. Kinase activities were normalized for levels of immunoreactive HA-Csk (means ± SEM). Expression of Cβ was also verified by immunoblotting. The data are representative of three independent experiments. (C) Csk activity was assessed in anti-HA immunoprecipitates of Jurkat TAg T cells transfected with HA-Csk-wt or mutant HA-Csk-S364C (means ± SEM). Cells were incubated in the presence or absence of 8-CPT-cAMP (300 μM for 40 min), and immunoprecipitations and kinase assays were performed as in B. Western blot analysis confirmed equal levels of Csk expression.
Figure 5
Mapping of Ser364 in Csk as a phosphorylation site for PKA in T cells. (A) Phosphoamino acid analysis of Csk phosphorylated by PKA. Csk (3 ng/μl) was incubated with PKA (GST-Cβ; 1.5 ng/μl active). (B) Wild-type (wt) Csk and mutant Csk-S364A were phosphorylated by Cα in vitro and subjected to tryptic peptide mapping (reference 41). (C) Tryptic peptide maps of 32Pi-Csk obtained by metabolic labeling of Jurkat T cells (reference 42). Endogenous Csk was immunoprecipitated from untreated, 8-CPT-cAMP–treated (300 μM for 30 min), or PGE1/IBMX-treated (10 μM/200 μg/ml for 5 min) cells. HA-tagged Csk or Csk-S364C was immunoprecipitated with an anti-HA mAb from transfected cells treated with 8-CPT-cAMP (300 μM for 30 min). To allow for production of enough material after excision of spots 1 and 2 for the rerunning experiment presented in D, higher levels of radioactivity were used in the experiment conducted with PGE1/IBMX-treated cells. (D) Peptides 1 and 2 from the PGE1-treated cells were excised from the gel and run again either alone (left panels) or mixed with peptides 1 and 2 from the experiment in Fig. 3 B (right panels; equal counts of each).
Figure 7
Pertubation of a PKA-Csk-Lck regulatory pathway mediates the inhibitory effect of cAMP on IL-2 secretion after T cell activation. (A) To establish a method for selecting only cells transfected with the gene of interest, Jurkat T cells were cotransfected with plasmids directing expression of a rat NK marker, NKR-P1A, and green fluorescent protein (GFP; left panel; 60% transfection efficiency, 47% double-positive cells), followed by separation of NKR-P1A positive (right) from negative (middle) cells using anti–NKR-P1 mAb and beads that allow detachment of cells after purification. Purity of the positive population was routinely 90–97%. (B) Anti-HA blot of cells transfected with HA-tagged Csk expression vectors and purified by bead selection for the cotransfected NKR-P1A. Negatively (−; lanes 1 and 3) and positively selected (+; lanes 2 and 4) cells are shown. (C) Jurkat T cells (clone E6.1) were transfected with Csk-wt expression vector at increasing doses of DNA, and the following day cells (6 × 105, 0.3 × 106/ml) positively selected for cotransfectant were stimulated by OKT3 (5 μg/ml) and PMA (10 nM) in the absence (•) or presence (○) of 8-CPT-cAMP (500 μM, 15 min pretreatment). After 20 h of culture, supernatants were harvested and analyzed for secreted IL-2. Pelleted cells were subjected to anti-Csk immunoblotting and densitometric scanning of levels of immunoreactive native and transfected (running with somewhat lower mobility) Csk. IL-2 levels were plotted relative to the ratio of transfected over native Csk. Arrow indicates the level of Csk expression used for the experiment in D. Representative of two experiments. (D) Positively selected Jurkat T cells expressing HA-Csk-wt or mutant HA-Csk-S364C at twofold above endogenous levels of Csk were stimulated and analyzed together with vector-transfected cells as in C for IL-2 secretion in the absence and presence of 8-CPT-cAMP. Percent inhibition of IL-2 secretion by cAMP for each cell culture is shown. In cells transfected with HA-Csk-wt, the total IL-2 production is inhibited both in the presence and absence of cAMP, whereas the ratio remains constant. Inset shows equal levels of transfected Csk-wt and Csk-S364C by anti-HA immunoblotting. Specific activities of wild-type and mutant Csk-S364C were comparable (data not shown). Representative of three experiments. (E) Jurkat T cells (five to six individual cell cultures) were transfected with empty vector (Control) or vectors directing expression of wild-type Lck and mutant Lck-Y505F that cannot be phosphorylated by Csk, stimulated as in C, and analyzed for IL-2 secretion (levels relative to stimulated are shown).
Figure 8
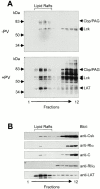
PKA and Csk are targeted to lipid rafts. Peripheral blood T cells were left untreated (−PV) or treated with pervanadate for 5 min (+PV) before homogenization in ice-cold lysis buffer with 1% Triton X-100 and separation in a 40–5% sucrose gradient. Fractions collected from the top 1 2 3 4 5 6 7 8 9 10 11 12 were analyzed by immunoblotting for (A) phosphotyrosine content and (B) distribution of Csk, PKA R and C subunits, and LAT. Mobility of molecular weight markers as well as of Cbp/PAG, Lck, and LAT are indicated in A. Blots in B represent parallel gel runs of the fractions in A (+PV). Observations are representative of three or more experiments.
Figure 9
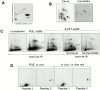
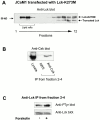
Forskolin stimulation of cells increases the phosphorylation of Y505 in Lck in lipid rafts. (A) Lck-deficient JCaM1 cells were transfected with a plasmid encoding catalytically inactive Lck-K273M. After harvesting, cells were homogenized in lysis buffer containing 0.7% Triton X-100 and subsequently separated in a 40–5% sucrose gradient. Fractions collected from the top 1 2 3 4 5 6 7 8 9 10 11 12 were analyzed by anti-Lck immunoblotting. Fractions 2–4 represent lipid raft fractions. Both transfected Lck-K273M and truncated catalytically inactive endogenous Lck are indicated. (B) Lipid raft fractions (fractions 2–4) from A were mixed and solubilized by addition of octyl-glucoside (50 mM). Thereafter, immunoprecipitation (IP) with either normal rabbit serum (NRS) or anti-Csk Abs was performed, and subsequent SDS-PAGE and anti-Csk immunoblotting were conducted. Triton X-100 lysate of JCaM1 cells is shown as control. (C) JCaM1 cells transfected with a plasmid encoding catalytically inactive Lck-K273M (same cells as in A) were incubated in the absence (−) or presence (+) of forskolin (100 μM) at 37°C for 10 min; thereafter lipid raft purification was performed as in A. Lipid raft fractions 2 3 4 were mixed and solubilized by the addition of octyl-glucoside (50 mM), and subjected to anti-Lck immunoprecipitation. After SDS-PAGE, the phosphotyrosine content of Lck was assessed by immunoblotting with antiphosphotyrosine Abs (4G10). Anti-Lck immunoblot (bottom) is shown as control. Densitometric analysis of both blots was conducted to assess the level of tyrosine phosphorylation of Lck-K273M.
Figure 10
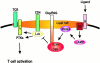
Model for a molecular mechanism whereby cAMP inhibits T cell function. Activation of adenylyl cyclase (AC), e.g., by binding of ligands such as adrenalin and PGE2 to G protein–coupled receptors (R), turns on a regulatory pathway assembled in lipid rafts that involves phosphorylation of Csk at S364 by PKA type I leading to activation of Csk. Activated Csk then phosphorylates and turns off Lck, and T cell activation is inhibited by the absence of ζ chain phosphorylation. Lipid raft association of Csk through interaction with phosphotyrosine Y314 in Cbp/Y317 in PAG and of PKA by interaction with A-kinase anchoring proteins and/or other docking proteins spatially facilitates modulation of T cell activation processes in lipid rafts by this inhibitory pathway.
Similar articles
- Removal of C-terminal SRC kinase from the immune synapse by a new binding protein.
Rahmouni S, Vang T, Alonso A, Williams S, van Stipdonk M, Soncini C, Moutschen M, Schoenberger SP, Mustelin T. Rahmouni S, et al. Mol Cell Biol. 2005 Mar;25(6):2227-41. doi: 10.1128/MCB.25.6.2227-2241.2005. Mol Cell Biol. 2005. PMID: 15743820 Free PMC article. - Combined spatial and enzymatic regulation of Csk by cAMP and protein kinase a inhibits T cell receptor signaling.
Vang T, Abrahamsen H, Myklebust S, Horejsí V, Taskén K. Vang T, et al. J Biol Chem. 2003 May 16;278(20):17597-600. doi: 10.1074/jbc.C300077200. Epub 2003 Mar 28. J Biol Chem. 2003. PMID: 12665526 - Knockdown of C-terminal Src kinase by siRNA-mediated RNA interference augments T cell receptor signaling in mature T cells.
Vang T, Abrahamsen H, Myklebust S, Enserink J, Prydz H, Mustelin T, Amarzguioui M, Tasken K. Vang T, et al. Eur J Immunol. 2004 Aug;34(8):2191-9. doi: 10.1002/eji.200425036. Eur J Immunol. 2004. PMID: 15259016 - Spatiotemporal control of cyclic AMP immunomodulation through the PKA-Csk inhibitory pathway is achieved by anchoring to an Ezrin-EBP50-PAG scaffold in effector T cells.
Cornez I, Taskén K. Cornez I, et al. FEBS Lett. 2010 Jun 18;584(12):2681-8. doi: 10.1016/j.febslet.2010.04.056. Epub 2010 Apr 24. FEBS Lett. 2010. PMID: 20420835 Review. - Negative regulation of T-cell receptor activation by the cAMP-PKA-Csk signalling pathway in T-cell lipid rafts.
Tasken K, Ruppelt A. Tasken K, et al. Front Biosci. 2006 Sep 1;11:2929-39. doi: 10.2741/2022. Front Biosci. 2006. PMID: 16720365 Review.
Cited by
- Targeting the hypoxia-adenosinergic signaling pathway to improve the adoptive immunotherapy of cancer.
Sitkovsky M, Ohta A. Sitkovsky M, et al. J Mol Med (Berl). 2013 Feb;91(2):147-55. doi: 10.1007/s00109-013-1001-9. Epub 2013 Jan 20. J Mol Med (Berl). 2013. PMID: 23334369 Free PMC article. Review. - Regulation of cytochrome c oxidase activity by c-Src in osteoclasts.
Miyazaki T, Neff L, Tanaka S, Horne WC, Baron R. Miyazaki T, et al. J Cell Biol. 2003 Mar 3;160(5):709-18. doi: 10.1083/jcb.200209098. J Cell Biol. 2003. PMID: 12615910 Free PMC article. - Prostaglandin E2 inhibits tumor necrosis factor-alpha RNA through PKA type I.
Stafford JB, Marnett LJ. Stafford JB, et al. Biochem Biophys Res Commun. 2008 Feb 1;366(1):104-9. doi: 10.1016/j.bbrc.2007.11.091. Epub 2007 Dec 3. Biochem Biophys Res Commun. 2008. PMID: 18060853 Free PMC article. - Genome-Wide Gene-Set Analysis Identifies Molecular Mechanisms Associated with ALS.
Vasilopoulou C, McDaid-McCloskey SL, McCluskey G, Duguez S, Morris AP, Duddy W. Vasilopoulou C, et al. Int J Mol Sci. 2023 Feb 16;24(4):4021. doi: 10.3390/ijms24044021. Int J Mol Sci. 2023. PMID: 36835433 Free PMC article. - A Population of Tumor-Infiltrating CD4+ T Cells Co-Expressing CD38 and CD39 Is Associated with Checkpoint Inhibitor Resistance.
Mitra A, Thompson B, Strange A, Amato CM, Vassallo M, Dolgalev I, Hester-McCullough J, Muramatsu T, Kimono D, Puranik AS, Weber JS, Woods D. Mitra A, et al. Clin Cancer Res. 2023 Oct 13;29(20):4242-4255. doi: 10.1158/1078-0432.CCR-23-0653. Clin Cancer Res. 2023. PMID: 37505479 Free PMC article.
References
- Mustelin T. T cell antigen receptor signalingthree families of tyrosine kinases and a phosphatase. Immunity. 1994;1:351–356. - PubMed
- Qian D., Weiss A. T cell antigen receptor signal transduction. Curr. Opin. Cell Biol. 1997;9:205–212. - PubMed
- Rudd C.E. Adaptors and molecular scaffolds in immune cell signaling. Cell. 1999;96:5–8. - PubMed
- Okada M., Nada S., Yamanashi Y., Yamamoto T., Nakagawa H. CSKa protein-tyrosine kinase involved in regulation of Src family kinases. J. Biol. Chem. 1991;266:24249–24252. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- AI41481/AI/NIAID NIH HHS/United States
- R01 AI035603/AI/NIAID NIH HHS/United States
- AI35603/AI/NIAID NIH HHS/United States
- AI48032/AI/NIAID NIH HHS/United States
- R01 AI048032/AI/NIAID NIH HHS/United States
- AI40552/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous