RhoC GTPase overexpression modulates induction of angiogenic factors in breast cells - PubMed (original) (raw)
RhoC GTPase overexpression modulates induction of angiogenic factors in breast cells
K L van Golen et al. Neoplasia. 2000 Sep-Oct.
Abstract
Inflammatory breast cancer (IBC) is a distinct and aggressive form of locally advanced breast cancer. IBC is highly angiogenic, invasive, and metastatic at its inception. Previously, we identified specific genetic alterations of IBC that contribute to this highly invasive phenotype. RhoC GTPase was overexpressed in 90% of archival IBC tumor samples, but not in stage-matched, non-IBC tumors. To study the role of RhoC GTPase in contributing to an IBC-like phenotype, we generated stable transfectants of human mammary epithelial cells overexpressing the RhoC gene, and studied the effect of RhoC GTPase overexpression on the modulation of angiogenesis in IBC. Levels of vascular endothelial growth factor (VEGF), basic fibroblast growth factor (bFGF), interleukin-6 (IL-6), and interleukin-8 (IL-8) were significantly higher in the conditioned media of the HME-RhoC transfectants than in the untransfected HME and HME-beta-galactosidase control media, similar to the SUM149 IBC cell line. Inhibition of RhoC function by introduction of C3 exotransferase decreased production of angiogenic factors by the HME-RhoC transfectants and the SUM149 IBC cell line, but did not affect the control cells. These data support the conclusion that overexpression of RhoC GTPase is specifically and directly implicated in the control of the production of angiogenic factors by IBC cells.
Figures
Figure 1
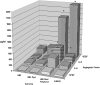
Comparison of levels of angiogenic factors by HME-RhoC transfectants, SUM149 IBC cell line and the HME-β-gal control cell line as determined by ELISA. The HME-RhoC cells produced significantly higher levels of angiogenic factors compared with the HME-β-gal control cell line, nearly recapitulating the levels produced by the SUM149 IBC cell line. Significant differences (p<0.001) between the control cells and the HME-RhoC cells are denoted by an asterisk (*) with standard deviations within 10% of the reported values.
Figure 2
Results of a rat aortic ring assay for functional angiogenic factors produced by untransfected HME (panel A), HME-β-gal control transfectants (panel B), HME-RhoC transfectants (panel C) and the SUM149 IBC cell line (panel D). Segments of rat aorta were embedded in Matrigel and cultured in the corresponding cell-conditioned media for 4 days and then observed for microvessel outgrowth. Conditioned media from the control cell lines (panels A and B) did not induce microvessel outgrowth. However, conditioned medium from the HME-RhoC transfectants (panel C) produced similar levels of new vessel growth as the SUM149 IBC cell line (panel D).
Figure 3
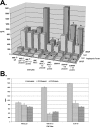
Panel A demonstrates the effect on production of angiogenic factors by inhibition of RhoC GTPase with C3 exotransferase. Similar levels of inhibition were accomplished by expressing a C3 exotransferase construct or introducing the active protein directly into the cells. Significant differences (p<0.05) between the untreated and C3 exotransferase treated cells are denoted by an asterisk (*) with standard deviations within 10% of the reported values. Panel B demonstrates the results of an in vitro ADP-ribosylation study to determine the in vivo efficiency of C3 exotransferase inhibition of Rho activity. The assay was performed as outlined in the Materials and Methods section. The potential ADP-ribosylated sites in both the HME-RhoC and SUM149 cell lines were significantly reduced after C3 treatment, thus indicating efficient in vivo inhibition of RhoC GTPase.
Figure 4
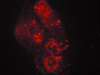
Rhodamine-labeled C3 exotransferase was introduced into cells using a lipid mediated transfer method (see Materials and Methods section). The presence and efficiency of C3 protein transfer was determined by visualizing cells under a fluorescent microscope.
Similar articles
- Mitogen activated protein kinase pathway is involved in RhoC GTPase induced motility, invasion and angiogenesis in inflammatory breast cancer.
van Golen KL, Bao LW, Pan Q, Miller FR, Wu ZF, Merajver SD. van Golen KL, et al. Clin Exp Metastasis. 2002;19(4):301-11. doi: 10.1023/a:1015518114931. Clin Exp Metastasis. 2002. PMID: 12090470 - WISP3 and RhoC guanosine triphosphatase cooperate in the development of inflammatory breast cancer.
Kleer CG, Zhang Y, Pan Q, Gallagher G, Wu M, Wu ZF, Merajver SD. Kleer CG, et al. Breast Cancer Res. 2004;6(1):R110-5. doi: 10.1186/bcr755. Breast Cancer Res. 2004. PMID: 14696649 Free PMC article. - Transfected MCF-7 cells as a model for breast-cancer progression.
Kern FG, McLeskey SW, Zhang L, Kurebayashi J, Liu Y, Ding IY, Kharbanda S, Chen D, Miller D, Cullen K, et al. Kern FG, et al. Breast Cancer Res Treat. 1994;31(2-3):153-65. doi: 10.1007/BF00666149. Breast Cancer Res Treat. 1994. PMID: 7881095 Review. - [Inflammatory breast carcinoma: towards molecular characterization?].
Charafe-Jauffret E, Tarpin C, Ginestier C, Bertucci F, Penault-Llorca F, Xerri L, Birnbaum D, Viens P, Hassoun J, Jacquemier J. Charafe-Jauffret E, et al. Ann Pathol. 2003 Dec;23(6):564-9. Ann Pathol. 2003. PMID: 15094594 Review. French.
Cited by
- RhoC in association with TET2/WDR5 regulates cancer stem cells by epigenetically modifying the expression of pluripotency genes.
Thomas P, Srivastava S, Udayashankara AH, Damodaran S, Yadav L, Mathew B, Suresh SB, Mandal AK, Srikantia N. Thomas P, et al. Cell Mol Life Sci. 2022 Dec 5;80(1):1. doi: 10.1007/s00018-022-04645-z. Cell Mol Life Sci. 2022. PMID: 36469134 Free PMC article. - Paracrine IL-6 Signaling Confers Proliferation between Heterogeneous Inflammatory Breast Cancer Sub-Clones.
Morrow RJ, Allam AH, Yeo B, Deb S, Murone C, Lim E, Johnstone CN, Ernst M. Morrow RJ, et al. Cancers (Basel). 2022 May 4;14(9):2292. doi: 10.3390/cancers14092292. Cancers (Basel). 2022. PMID: 35565421 Free PMC article. - Pathological and molecular characteristics of inflammatory breast cancer.
Di Bonito M, Cantile M, Botti G. Di Bonito M, et al. Transl Cancer Res. 2019 Oct;8(Suppl 5):S449-S456. doi: 10.21037/tcr.2019.03.24. Transl Cancer Res. 2019. PMID: 35117122 Free PMC article. Review. - RhoC Modulates Cell Junctions and Type I Interferon Response in Aggressive Breast Cancers.
Abraham HG, Ulintz PJ, Goo L, Yates JA, Little AC, Bao L, Wu Z, Merajver SD. Abraham HG, et al. Front Oncol. 2021 Aug 26;11:712041. doi: 10.3389/fonc.2021.712041. eCollection 2021. Front Oncol. 2021. PMID: 34513691 Free PMC article. - Rho GTPases as Key Molecular Players within Intestinal Mucosa and GI Diseases.
Pradhan R, Ngo PA, Martínez-Sánchez LD, Neurath MF, López-Posadas R. Pradhan R, et al. Cells. 2021 Jan 4;10(1):66. doi: 10.3390/cells10010066. Cells. 2021. PMID: 33406731 Free PMC article. Review.
References
- Levine PH, Steinhorn SC, Ries IG. Inflammatory breast cancer. The experience of the surveillance, epidemiology, and end results (SEER) program. J Natl Cancer Inst. 1985;74:291–297. - PubMed
- Jaiyesimi I, Buzdar A, Hortobagyi G. Inflammatory breast cancer: a review. J Clin Oncol. 1992;10:1014–1024. - PubMed
- Beahrs O, Henson D, Hutter R. Manual for Staging of Cancer. (3rd ed) 1988:145–150.
- van Golen KL, Davies S, Wu ZF, Wang Y, Bucana CD, Root H, Chandrasekharappa S, Strawderman M, Ethier SP, Merajver SD. A novel putative low-affinity insulin-like growth factor-binding protein, LIBC (lost in inflammatory breast cancer), and RhoC GTPase correlate with the inflammatory breast cancer phenotype. Clin Cancer Res. 1999;5:2511–2519. - PubMed
- van Golen KL, Wu ZF, Qiao XT, Bao LW, Merajver SD. RhoC GTPase, a novel transforming oncogene for human mammary epithelial cells that partially recapitulates the inflammatory breast cancer phenotype. Cancer Res. 2000;60:5832–5838. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 CA077612/CA/NCI NIH HHS/United States
- T32 CA009537/CA/NCI NIH HHS/United States
- 5T32 CA09537 - 16/CA/NCI NIH HHS/United States
- R01 CA 77612/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous