SH2-B and APS are multimeric adapters that augment TrkA signaling - PubMed (original) (raw)
SH2-B and APS are multimeric adapters that augment TrkA signaling
X Qian et al. Mol Cell Biol. 2001 Mar.
Abstract
Neurotrophins influence growth and survival of sympathetic and sensory neurons through activation of their receptors, Trk receptor tyrosine kinases. Previously, we identified Src homology 2-B (SH2-B) and APS, which are structurally similar adapter proteins, as substrates of Trk kinases. In the present study, we demonstrate that both SH2-B and APS exist in cells as homopentamers and/or heteropentamers, independent of Trk receptor activation. Structure-function analyses revealed that the SH2-B multimerization domain resides within its amino terminus, which is necessary for SH2-B-mediated nerve growth factor (NGF) signaling. Overexpression of SH2-B enhances both the magnitude and duration of TrkA autophosphorylation following exposure of PC12 cells to NGF, and this effect requires the amino-terminal multimerization motif. Moreover, the amino terminus of SH2-B is necessary for TrkA/SH2-B-mediated morphological differentiation of PC12 cells. Together, these results indicate that the multimeric adapters SH2-B and APS influence neurotrophin signaling through direct modulation of Trk receptor autophosphorylation.
Figures
FIG. 1
SH2-B and APS exist as homomultimers or heteromultimers in neurons. (A) Molecular structure of SH2-B and APS. SH2-B and APS were previously identified as Trk interactors (15) which contain several well-conserved domains, including SH2 and PH domains. Percent amino acid identity is indicated. (B) SH2-B heteromultimerizes with APS in neurons. Cortical neurons from E18 rats were untreated or treated with BDNF (50 ng/ml, 10 min), which activates TrkB. Cell lysates were prepared and subjected to immunoprecipitation (IP) using either SH2-B or APS antibodies. These antibodies do not cross-react (data not shown). Immune complexes were then resolved by SDS-PAGE and immunoblotted with an anti-human APS antibody (Santa Cruz) which recognizes both APS and SH2-B. (C) SH2-B forms homomultimers and heteromultimerizes with APS. Myc- and HA-tagged SH2-B and APS were transiently expressed in HEK293T cells. Cells were unstimulated or stimulated with NGF (100 ng/ml, 10 min). Cell lysates were prepared and subjected to immunoprecipitation (IP) using an anti-Myc antibody. Immune complexes were resolved by SDS-PAGE and subjected to immunoblotting with either anti-HA (top) or anti-Myc (bottom) antibodies. Sizes are shown in kilodaltons in this and subsequent figures. rAPS, rat APS.
FIG. 2
SH2-B homomultimerizes through an amino-terminal association domain. (A) Schematic diagram of SH2-B deletion and tyrosine mutants. Full-length SH2-B and mutants containing an amino-terminal Myc epitope tag were cloned into mammalian expression vector pRK5. AA, amino acids. (B, C, and D) The amino-terminal region of SH2-B is both necessary and sufficient for SH2-B multimerization. Myc-tagged SH2-B mutants depicted in panel A were coexpressed with HA-tagged SH2-B in HEK293T cells. Cell extracts were subjected to immunoprecipitation (IP) with anti-Myc antibody. The immune complexes were then resolved by SDS-PAGE and immunoblotted using anti-Myc or anti-HA antibodies.
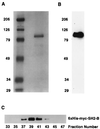
FIG. 3
SH2-B and APS exist as large complexes, as determined by size exclusion chromatography. (A) Standard molecular weight (MW) curve for size exclusion chromatography (Sepharose 12; Pharmacia). BSA, bovine serum albumin. (B) Lysates of HEK293T cells expressing either Myc-SH2-B or Myc-APS were fractionated by size exclusion chromatography, and samples from fractions were resolved by SDS-PAGE and immunoblotted using the anti-Myc antibody. (C) Nondenatured cell lysates prepared from primary cortical neurons were fractionated, and fractions were resolved by SDS-PAGE and immunoblotted with either anti-APS or anti-SH2-B antibodies. The SH2-B and APS complexes elute in fractions 36 to 42 and 38 to 44, which correspond to molecular masses of approximately 440 and 350 kDa, respectively. rAPS, rat APS.
FIG. 4
SH2-B is the major component of the SH2-B-containing complex. Cell lysates prepared from HEK293T cells expressing 6×His-Myc-SH2-B were prepared and incubated with Ni-NTA resin. After incubation, the 6×His-Myc-SH2-B complex was eluted with a buffer containing imidazole (250 mM, pH 6.5). The eluent was dialyzed against PBS and concentrated. The purified 6×His-Myc-SH2-B was resolved by SDS-PAGE and either stained with Coomassie blue (A) or immunoblotted with anti-Myc (B). The purified 6×His-Myc-SH2-B complex was fractionated by size exclusion chromatography, and fractions were resolved by SDS-PAGE and immunoblotted with anti-Myc (C).
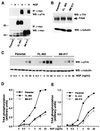
FIG. 5
SH2-B enhances NGF-induced tyrosine phosphorylation of TrkA in PC12 cells. (A) Parental PC12 cells or PC12 cells stably expressing either full-length SH2-B or the truncated M8 mutant were unstimulated or stimulated with NGF (100 ng/ml, 10 min). Cell lysates were prepared and subjected to immunoprecipitation (IP) using a Myc antibody. The immune complexes were resolved by SDS-PAGE and Western blotted (WB) using the pTrk antibody (upper panel) or anti-Myc (lower panel). (B) Cell lysates prepared from parental PC12 cells or PC12 cells stably expressing either full-length SH2-B or M8 were separated by SDS-PAGE and Western blotted (WB) with either a pan-Trk antibody (upper panel) or an antitubulin antibody. (C) Parental PC12 cells or PC12 cells stably expressing either full-length SH2-B (FL #65) or M8 (M8 #17) were stimulated with NGF at a range of concentrations as indicated. Cell extracts were resolved by SDS-PAGE and Western blotted (WB) with the pTrk antibody, and the blot was stripped and reprobed with antitubulin. The phosphorylated TrkA and tubulin signals were quantified using Image Storm analysis and the phospho-TrkA and tubulin levels illustrated in panel D. Similar experiments were performed using another set of stable PC12 clones expressing either full-length SH2-B (FL #15) or M8 (M8 #16). Quantitation of the level of phosphorylation of TrkA receptor normalized to amounts of tubulin is shown in panel E.
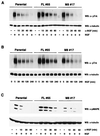
FIG. 6
SH2-B modulates the kinetics of NGF-induced phosphorylation of both TrkA and MAP kinase (MAPK) in PC12 cells. Parental PC12 cells or PC12 cells stably expressing either full-length SH2-B or M8 were stimulated with a pulse of NGF (100 ng/ml, 10 min [A] or 3 ng/ml, 10 min [C]). Then, medium was replaced with fresh medium containing a function blocking NGF antibody (A and C). In the experiments shown in B, cells were exposed to NGF (100 ng/ml) continuously for the indicated times. Cell extracts were prepared and resolved by SDS-PAGE and Western blotted (WB) with the indicated antibodies. Similar results were observed with experiments using other SH2-B and M8 clones (data not shown).
FIG. 7
Amino terminus of SH2-B modulates NGF-mediated morphological differentiation of PC12 cells. (A) The SH2-B amino-terminal domain is necessary for NGF-induced differentiation of PC12nnr5 cells. Either full-length SH2-B or one of several SH2-B mutants was transiently expressed in PC12nnr5 cells along with the TrkA mutant F8 and GFP. Cells were treated with NGF (100 ng/ml) for 3 days after transfection. Cells were then fixed, and neurite outgrowth of GFP-positive cells was scored. The TrkA mutant F8 has mutations in all conserved tyrosines except Y670 and Y674/5 (8). This mutant cannot bind to Shc and phospholipase C-γ and cannot support morphological differentiation of PC12nnr5 cells unless coexpressed with SH2-B or APS (15). Vect, vector. (B) PC12 cells expressing full-length SH2-B are more responsive to NGF than either parental PC12 cells or PC12 cells expressing M8. Parental PC12 cells or PC12 cell clones stably expressing either full-length SH2-B (FL #15 and FL #65) or M8 (M8 #16 and M8 #17) were stimulated with the indicated concentrations of NGF for 3 days and then fixed and scored for neurite outgrowth. The results shown were from three independent experiments.
Similar articles
- Identification and characterization of novel substrates of Trk receptors in developing neurons.
Qian X, Riccio A, Zhang Y, Ginty DD. Qian X, et al. Neuron. 1998 Nov;21(5):1017-29. doi: 10.1016/s0896-6273(00)80620-0. Neuron. 1998. PMID: 9856458 - SH2-B is required for nerve growth factor-induced neuronal differentiation.
Rui L, Herrington J, Carter-Su C. Rui L, et al. J Biol Chem. 1999 Apr 9;274(15):10590-4. doi: 10.1074/jbc.274.15.10590. J Biol Chem. 1999. PMID: 10187854 - Molecular dissection of TrkA signal transduction pathways mediating differentiation in human neuroblastoma cells.
Eggert A, Ikegaki N, Liu X, Chou TT, Lee VM, Trojanowski JQ, Brodeur GM. Eggert A, et al. Oncogene. 2000 Apr 13;19(16):2043-51. doi: 10.1038/sj.onc.1203518. Oncogene. 2000. PMID: 10803465 - The FRK/RAK-SHB signaling cascade: a versatile signal-transduction pathway that regulates cell survival, differentiation and proliferation.
Annerén C, Lindholm CK, Kriz V, Welsh M. Annerén C, et al. Curr Mol Med. 2003 Jun;3(4):313-24. doi: 10.2174/1566524033479744. Curr Mol Med. 2003. PMID: 12776987 Review. - Functional effects of APS and SH2-B on insulin receptor signalling.
Ahmed Z, Pillay TS. Ahmed Z, et al. Biochem Soc Trans. 2001 Aug;29(Pt 4):529-34. doi: 10.1042/bst0290529. Biochem Soc Trans. 2001. PMID: 11498022 Review.
Cited by
- Phosphorylation controls a dual-function polybasic nuclear localization sequence in the adapter protein SH2B1β to regulate its cellular function and distribution.
Maures TJ, Su HW, Argetsinger LS, Grinstein S, Carter-Su C. Maures TJ, et al. J Cell Sci. 2011 May 1;124(Pt 9):1542-52. doi: 10.1242/jcs.078949. Epub 2011 Apr 12. J Cell Sci. 2011. PMID: 21486950 Free PMC article. - The adaptor protein SH2B3 (Lnk) negatively regulates neurite outgrowth of PC12 cells and cortical neurons.
Wang TC, Chiu H, Chang YJ, Hsu TY, Chiu IM, Chen L. Wang TC, et al. PLoS One. 2011;6(10):e26433. doi: 10.1371/journal.pone.0026433. Epub 2011 Oct 18. PLoS One. 2011. PMID: 22028877 Free PMC article. - SH2B1 regulation of energy balance, body weight, and glucose metabolism.
Rui L. Rui L. World J Diabetes. 2014 Aug 15;5(4):511-26. doi: 10.4239/wjd.v5.i4.511. World J Diabetes. 2014. PMID: 25126397 Free PMC article. Review. - The SH2B1 adaptor protein associates with a proximal region of the erythropoietin receptor.
Javadi M, Hofstätter E, Stickle N, Beattie BK, Jaster R, Carter-Su C, Barber DL. Javadi M, et al. J Biol Chem. 2012 Jul 27;287(31):26223-34. doi: 10.1074/jbc.M112.382721. Epub 2012 Jun 5. J Biol Chem. 2012. PMID: 22669948 Free PMC article. - Mutation screen in the GWAS derived obesity gene SH2B1 including functional analyses of detected variants.
Volckmar AL, Bolze F, Jarick I, Knoll N, Scherag A, Reinehr T, Illig T, Grallert H, Wichmann HE, Wiegand S, Biebermann H, Krude H, Fischer-Posovszky P, Rief W, Wabitsch M, Klingenspor M, Hebebrand J, Hinney A. Volckmar AL, et al. BMC Med Genomics. 2012 Dec 27;5:65. doi: 10.1186/1755-8794-5-65. BMC Med Genomics. 2012. PMID: 23270367 Free PMC article.
References
- Barker P. Nerve growth factor and the low-affinity neurotrophin receptor. In: Sieber-Blum M, editor. Neurotrophins and the neural crest. Boca Raton, Fla: CRC Press; 1998. pp. 59–93.
- Brakeman P R, Lanahan A A, O'Brien R, Roche K, Barnes C A, Huganir R L, Worley P F. Homer, a protein that selectively binds to metabotrophic glutamate receptors. Nature. 1997;386:221–223. - PubMed
- Cunningham M E, Stephens R M, Kaplan D R, Greene L A. Autophosphorylation of activation loop tyrosines regulates signaling by the TRK nerve growth factor receptor. J Biol Chem. 1997;272:10957–10967. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous