Segregation of heterotrimeric G proteins in cell surface microdomains. G(q) binds caveolin to concentrate in caveolae, whereas G(i) and G(s) target lipid rafts by default - PubMed (original) (raw)
Segregation of heterotrimeric G proteins in cell surface microdomains. G(q) binds caveolin to concentrate in caveolae, whereas G(i) and G(s) target lipid rafts by default
P Oh et al. Mol Biol Cell. 2001 Mar.
Free PMC article
Abstract
Select lipid-anchored proteins such as glycosylphosphatidylinositol (GPI)-anchored proteins and nonreceptor tyrosine kinases may preferentially partition into sphingomyelin-rich and cholesterol-rich plasmalemmal microdomains, thereby acquiring resistance to detergent extraction. Two such domains, caveolae and lipid rafts, are morphologically and biochemically distinct, contain many signaling molecules, and may function in compartmentalizing cell surface signaling. Subfractionation and confocal immunofluorescence microscopy reveal that, in lung tissue and in cultured endothelial and epithelial cells, heterotrimeric G proteins (G(i), G(q), G(s), and G(betagamma)) target discrete cell surface microdomains. G(q) specifically concentrates in caveolae, whereas G(i) and G(s) concentrate much more in lipid rafts marked by GPI-anchored proteins (5' nucleotidase and folate receptor). G(q), apparently without G(betagamma) subunits, stably associates with plasmalemmal and cytosolic caveolin. G(i) and G(s) interact with G(betagamma) subunits but not caveolin. G(i) and G(s), unlike G(q), readily move out of caveolae. Thus, caveolin may function as a scaffold to trap, concentrate, and stabilize G(q) preferentially within caveolae over lipid rafts. In N2a cells lacking caveolae and caveolin, G(q), G(i), and G(s) all concentrate in lipid rafts as a complex with G(betagamma). Without effective physiological interaction with caveolin, G proteins tend by default to segregate in lipid rafts. The ramifications of the segregated microdomain distribution and the G(q)-caveolin complex without G(betagamma) for trafficking, signaling, and mechanotransduction are discussed.
Figures
Figure 1
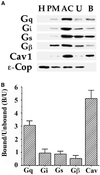
Distribution of G proteins in isolated membranes and caveolin-coated caveolae. (A) Subcellular fractionation was performed on rat lung tissues to obtain whole lung homogenates (H), silica-coated luminal endothelial cell plasma membranes (P), caveolae (V), and repelleted silica-coated membranes stripped of caveolae (P-V). Western analysis of 5 μg of each these fractions is shown using antibodies to Gi, Gs, Gq, and Gβ, 5′ NT, ε-Cop, and caveolin-1 and 2. (B) The caveolae fraction (V) was subjected to immuno-affinity isolation using magnetic beads attached to antibodies to caveolin-1 (cav) or clathrin heavy chain (clath). Five micrograms of the V fraction starting material (SM) and the entire amount of antibody bound (B) or unbound (U) material was subjected to Western analysis using antibodies to the indicated proteins.
Figure 2
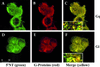
Immunofluorescence microscopy of G protein and caveolin-1 colocalization. Bovine lung microvascular endothelial cells (BLMVEC) were fixed and incubated with antibodies, either to caveolin (left panel, red labeling) or to the indicated G proteins (middle panel, green labeling). Signal overlap for caveolin and G protein generates yellow in the overlay of the two images (right panel).
Figure 3
Differential distribution of G proteins in lipid rafts. Caveolae (V) and lipid rafts (LR) were isolated from the silica-coated endothelial cell plasma membranes (P) derived from lung. Western analysis of V and LR (5 μg each) is shown using antibodies to the indicated G proteins, caveolin-1, and the lipid raft marker, 5′NT.
Figure 4
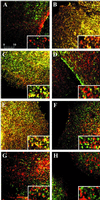
Immunofluorescence microscopy of lipid rafts for colocalization with G proteins and caveolin. MA104 cells (panels A-G) were grown on coverslips, fixed, and incubated with monoclonal antibodies to either folate receptor (panels A, C, E, green labeling) or caveolin (panels B, D, F; green labeling), and polyclonal antibodies to Gq (panels A and B, red/orange), Gi (panels C and D, red/orange), Gs (panel E and F, red/orange), and caveolin (G, red/orange). (Panel H) BLMVEC labeled with 5′NT antibodies (green) and caveolin antibodies (red/orange). Images were overlaid, and the signal overlap is indicated in yellow. Inset: higher magnification of a representative area of the image showing overlapping signals. Magnification of insets, 3×.
Figure 5
Western analysis of sonicated low-density caveolin-rich fractions and immuno-isolated caveolae. (A) Rat lung tissue homogenates were fractionated on a Percoll gradient to isolate plasma membranes (lane PM), followed by sonication and sucrose gradient centrifugation to isolate a caveolin-rich fraction (lane AC). Subsequently, 25 μg of AC was subjected to immuno-affinity isolation using caveolin-1 antibody conjugated magnetic beads separate caveolin-coated vesicles bound to the beads (B) from noncaveolar unbound material (U) in the supernatant. Western analysis was performed on 5 μg of each fraction using antibodies to the indicated proteins. (B) The level of specific proteins in the bound versus the unbound fraction was quantified, and the ratiowas plotted for caveolin and for each of the indicated G proteins.
Figure 6
Immunofluorescence microscopy of Gq and Gi concentrated in lipid rafts of N2a cells lacking caveolin and caveolae. N2a cells were fixed and incubated with monoclonal antibodies to 5′NT (panels A, D, green labeling) and polyclonal antibodies to Gq (panel B, red) and Gi (panel E, red). Images were overlaid (panels C and F), and the signal overlap is indicated in yellow. Inset: higher magnification of a representative area of the image showing overlapping signals.
Figure 7
Coimmunoprecipitation of G proteins with membrane-bound caveolin. Proteins solubilized from plasma membranes (P) were subjected to immunoprecipitation (IP) using antibodies to the indicated proteins bound to magnetic beads. The starting material (SM) (5 μg) solubilized from P and equal volumes of the material bound to the beads (I) or unbound in the supernatant (S) were subjected to Western analysis using antibodies to the indicated proteins.
Figure 8
Coimmunoprecipitation of Gq with soluble caveolin. Cytosols were prepared from rat lung homogenates and subjected to immunoprecipitation (IP) with magnetic beads coated with caveolin-1 or G protein antibodies as indicated. Equal volumes of the material bound to the beads was then subjected to Western analysis (WB), using antibodies to caveolin-1 or the indicated G proteins. HC, immunoglobulin heavy chain; LC, immunoglobulin light chain.
Figure 9
Coimmunoprecipitation of Gαβγ complexes in N2a cells lacking caveolae. Proteins solubilized from N2a cells were subjected to immunoprecipitation (IP) using antibodies to the indicated proteins bound to magnetic beads. The starting material (SM) (5 μg) and equal volumes of the material bound to the beads (I) or unbound in the supernatant (S) were subjected to Western analysis using antibodies to Gq.
Figure 10
Dissociation of G proteins from caveolae. Isolated caveolae (V) (20 μg) were diluted in MBS and gently mixed at 4°C for 4 h followed by centrifugation to separate the repelleted caveolar membranes (R) from the soluble phase containing any dissociated proteins (D). An equal volume of starting caveolae (lane SM), R, and D were subjected to Western analysis using antibodies to the indicated proteins.
Similar articles
- Caveolin-1 and lipid microdomains regulate Gs trafficking and attenuate Gs/adenylyl cyclase signaling.
Allen JA, Yu JZ, Dave RH, Bhatnagar A, Roth BL, Rasenick MM. Allen JA, et al. Mol Pharmacol. 2009 Nov;76(5):1082-93. doi: 10.1124/mol.109.060160. Epub 2009 Aug 20. Mol Pharmacol. 2009. PMID: 19696145 Free PMC article. - Sphingolipid-cholesterol domains (lipid rafts) in normal human and dog thyroid follicular cells are not involved in thyrotropin receptor signaling.
Costa MJ, Song Y, Macours P, Massart C, Many MC, Costagliola S, Dumont JE, Van Sande J, Vanvooren V. Costa MJ, et al. Endocrinology. 2004 Mar;145(3):1464-72. doi: 10.1210/en.2003-1432. Epub 2003 Dec 11. Endocrinology. 2004. PMID: 14670987 - Co-purification and direct interaction of Ras with caveolin, an integral membrane protein of caveolae microdomains. Detergent-free purification of caveolae microdomains.
Song KS, Li Shengwen, Okamoto T, Quilliam LA, Sargiacomo M, Lisanti MP. Song KS, et al. J Biol Chem. 1996 Apr 19;271(16):9690-7. doi: 10.1074/jbc.271.16.9690. J Biol Chem. 1996. PMID: 8621645 - Caveolins, caveolae, and lipid rafts in cellular transport, signaling, and disease.
Quest AF, Leyton L, Párraga M. Quest AF, et al. Biochem Cell Biol. 2004 Feb;82(1):129-44. doi: 10.1139/o03-071. Biochem Cell Biol. 2004. PMID: 15052333 Review. - [Role of lipid rafts in trimeric G protein-mediated signal transduction].
Ohkubo S, Nakahata N. Ohkubo S, et al. Yakugaku Zasshi. 2007 Jan;127(1):27-40. doi: 10.1248/yakushi.127.27. Yakugaku Zasshi. 2007. PMID: 17202782 Review. Japanese.
Cited by
- Phosphatidylinositol-3,4,5-trisphosphate: tool of choice for class I PI 3-kinases.
Salamon RS, Backer JM. Salamon RS, et al. Bioessays. 2013 Jul;35(7):602-11. doi: 10.1002/bies.201200176. Bioessays. 2013. PMID: 23765576 Free PMC article. Review. - The role of beta(1)Pix/caveolin-1 interaction in endothelin signaling through Galpha subunits.
Chahdi A, Sorokin A. Chahdi A, et al. Biochem Biophys Res Commun. 2010 Jan 15;391(3):1330-5. doi: 10.1016/j.bbrc.2009.12.041. Epub 2009 Dec 21. Biochem Biophys Res Commun. 2010. PMID: 20026011 Free PMC article. - Glycosylphosphatidyl inositol-anchored proteins and fyn kinase assemble in noncaveolar plasma membrane microdomains defined by reggie-1 and -2.
Stuermer CA, Lang DM, Kirsch F, Wiechers M, Deininger SO, Plattner H. Stuermer CA, et al. Mol Biol Cell. 2001 Oct;12(10):3031-45. doi: 10.1091/mbc.12.10.3031. Mol Biol Cell. 2001. PMID: 11598189 Free PMC article. - Rafting on the Plasma Membrane: Lipid Rafts in Signaling and Disease.
Isik OA, Cizmecioglu O. Isik OA, et al. Adv Exp Med Biol. 2023;1436:87-108. doi: 10.1007/5584_2022_759. Adv Exp Med Biol. 2023. PMID: 36648750 Review. - Lipid rafts: heterogeneity on the high seas.
Pike LJ. Pike LJ. Biochem J. 2004 Mar 1;378(Pt 2):281-92. doi: 10.1042/BJ20031672. Biochem J. 2004. PMID: 14662007 Free PMC article. Review.
References
- Ahmed SN, Brown DA, London E. On the origin of sphingolipid/cholesterol-rich detergent-insoluble cell membranes: physiological concentrations of cholesterol and sphingolipid induce formation of a detergent-insoluble, liquid-ordered lipid phase in model membranes. Biochem. 1997;36:10944–10953. - PubMed
- Brown DA, Rose JK. Sorting of GPI-anchored proteins to glycolipid-enriched membrane subdomains during transport to the apical cell surface. Cell. 1992;68:533–544. - PubMed
- Brown DA, London E. Structure and function of sphingolipid- and cholesterol-rich membrane rafts. J Biol Chem. 2000;275:17221–17224. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials