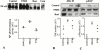Attenuation of colon inflammation through activators of the retinoid X receptor (RXR)/peroxisome proliferator-activated receptor gamma (PPARgamma) heterodimer. A basis for new therapeutic strategies - PubMed (original) (raw)
. 2001 Apr 2;193(7):827-38.
doi: 10.1084/jem.193.7.827.
L Dubuquoy, S Nutten, M Peuchmaur, W Englaro, K Schoonjans, B Derijard, B Desvergne, W Wahli, P Chambon, M D Leibowitz, J F Colombel, J Auwerx
Affiliations
- PMID: 11283155
- PMCID: PMC2193371
- DOI: 10.1084/jem.193.7.827
Attenuation of colon inflammation through activators of the retinoid X receptor (RXR)/peroxisome proliferator-activated receptor gamma (PPARgamma) heterodimer. A basis for new therapeutic strategies
P Desreumaux et al. J Exp Med. 2001.
Abstract
The peroxisome proliferator-activated receptor gamma (PPARgamma) is highly expressed in the colon mucosa and its activation has been reported to protect against colitis. We studied the involvement of PPARgamma and its heterodimeric partner, the retinoid X receptor (RXR) in intestinal inflammatory responses. PPARgamma(1/)- and RXRalpha(1/)- mice both displayed a significantly enhanced susceptibility to 2,4,6-trinitrobenzene sulfonic acid (TNBS)-induced colitis compared with their wild-type littermates. A role for the RXR/PPARgamma heterodimer in the protection against colon inflammation was explored by the use of selective RXR and PPARgamma agonists. TNBS-induced colitis was significantly reduced by the administration of both PPARgamma and RXR agonists. This beneficial effect was reflected by increased survival rates, an improvement of macroscopic and histologic scores, a decrease in tumor necrosis factor alpha and interleukin 1beta mRNA levels, a diminished myeloperoxidase concentration, and reduction of nuclear factor kappaB DNA binding activity, c-Jun NH(2)-terminal kinase, and p38 activities in the colon. When coadministered, a significant synergistic effect of PPARgamma and RXR ligands was observed. In combination, these data demonstrate that activation of the RXR/PPARgamma heterodimer protects against colon inflammation and suggest that combination therapy with both RXR and PPARgamma ligands might hold promise in the clinic due to their synergistic effects.
Figures
Figure 1
Design of the animal intervention studies. TNBS (black ellipse) was administered intrarectally on day (D) 0 and mice were killed 2 or 5 d later. The rexinoid (LG101305), the PPARγ agonists, such as rosiglitazone and troglitazone, or the combination of a rexinoid and a PPARγ agonist were administered at the indicated doses 2 d before the induction of colitis (preventive mode). The therapeutic effects were evaluated in mice receiving the same dose of rosiglitazone just after TNBS administration (therapeutic mode). Treatment with PPARγ and/or RXR agonists was evaluated at day 2 or 5 by scoring for mortality, determination of macroscopic and histologic inflammation scores, and measurements of inflammatory parameters (MPO levels, TNF-α and IL-1β mRNA, NF-κB pathway, and MAPK activity).
Figure 2
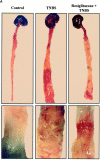
TNBS-induced colitis is dose-dependently improved by PPARγ agonists. (A) Macroscopic appearance of the colon of mice receiving vehicle only (Control), TNBS, or mice receiving rosiglitazone (20 mg/kg/d) 2 d before the administration of TNBS (Rosiglitazone + TNBS). The bottom shows a larger magnification of the colon at the injection site. (B and C) The antiinflammatory effects of different doses of troglitazone (B) and rosiglitazone (C) were assessed preventively in TNBS-induced colitis. The severity of the lesions was evaluated by macroscopic and histologic assessments using, respectively, the Wallace and Ameho scores in mice killed 2 d after colitis induction.
Figure 2
TNBS-induced colitis is dose-dependently improved by PPARγ agonists. (A) Macroscopic appearance of the colon of mice receiving vehicle only (Control), TNBS, or mice receiving rosiglitazone (20 mg/kg/d) 2 d before the administration of TNBS (Rosiglitazone + TNBS). The bottom shows a larger magnification of the colon at the injection site. (B and C) The antiinflammatory effects of different doses of troglitazone (B) and rosiglitazone (C) were assessed preventively in TNBS-induced colitis. The severity of the lesions was evaluated by macroscopic and histologic assessments using, respectively, the Wallace and Ameho scores in mice killed 2 d after colitis induction.
Figure 3
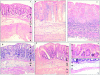
Representative histological sections of colon tissues of Balb/c mice. (A) Normal transparietal colon section of a vehicle-treated mouse with an Ameho score of 0 (×250). The different layers are indicated: M, mucosa; SM, submucosa; Mu, muscular layer. (B) Transparietal colon section (Ameho score 6) 2 d after the induction of colitis by TNBS. Thickening of the colon wall, with a predominant inflammatory infiltrate in the lamina propria, and necrosis extending deeply into the muscular and serosal layers are evident (×400). (C) Transparietal colon section (Ameho score 6) 5 d after the induction of colitis by TNBS. Parietal necrosis extending deeply into the muscular layer with the disappearance of cells in the mucosa is visible (×250). (D) Transparietal colon section of a mouse, which received rosiglitazone before TNBS administration. The mouse was killed 2 d after colitis induction. The Ameho score was graded 2. The picture shows a subepithelial edema with a diastasis of the crypts and a moderate inflammatory infiltrate in the mucosa and submucosa (×250). (E) Transparietal colonic section of mice treated with rosiglitazone after administration of TNBS. The pictures show mice killed 5 d after colitis induction. Ulceration extending into the submucosa, associated with a mucosal, submucosal, and muscular inflammatory infiltrate involving <50% of the specimen, is visible (×250). (F and G) Colon sections of mice that received rosiglitazone before TNBS administration. The mice were killed 2 d after colitis induction. In some cases, a total repair of the mucosa was observed (F; ×600) despite the persistence of an in-depth focal necrosis in the submucosal layer (G; ×400).
Figure 4
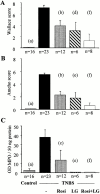
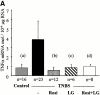
Effect of PPARγ and RXR agonists on TNBS-induced colitis. Wallace macroscopic inflammation score (A), Ameho histologic score (B), and colon MPO levels (C) of mice receiving vehicle only (Control), TNBS, rosiglitazone (Rosi at 20 mg/kg/d), LG101305 (LG at 20 mg/kg/d), or rosiglitazone and LG101305 simultaneously (Rosi + LG both at 20 mg/kg/d) 2 d before the administration of TNBS. The number of mice is indicated and results are expressed as the mean ± SEM. Animals were killed 2 d after TNBS treatment. (a) P < 0.001 in control mice vs. untreated TNBS colitis; (b) P < 0.001 and (c) P = 0.009 in untreated TNBS colitis vs. mice receiving rosiglitazone; (d) P = 0.002 and (e) P < 0.001 in untreated TNBS colitis vs. mice receiving LG101305; and (f) P < 0.001 in untreated TNBS colitis vs. mice receiving both rosiglitazone and LG101305.
Figure 5
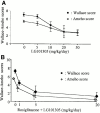
PPARγ-RXR agonists have a synergistic effect on colitis. The antiinflammatory effects of different doses of the RXR agonist LG101305 (A) and of the simultaneous administrations of rosiglitazone (from 1 to 20 mg/kg/d) and LG101305 (from 1 to 20 mg/kg/d)(B) were assessed in TNBS-induced colitis. The severity of the lesions was evaluated by macroscopic and histologic assessments using, respectively, the Wallace and Ameho scores in mice killed 2 d after colitis induction.
Figure 6
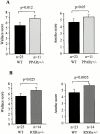
PPARγ1/− and RXRα1/− mice are more susceptible to TNBS-induced colitis. (A and B) Wallace macroscopic and Ameho histologic inflammation scores of 129/Sv wild-type (WT), PPARγ1/− (A) and RXRα1/− (B) mice 2 d after induction of colitis by TNBS administration. (C) Representative transparietal colon section of 129/Sv wild-type mice (Ameho score 5) 2 d after the induction of colitis by TNBS showing a moderate infiltrate with a necrosis limited to the superficial part of the mucosa (×200). (D and E) Transparietal colon sections in PPARγ1/− (D) and RXRα1/− (E) mice (both Ameho scores of 6) 2 d after the induction of colitis by TNBS. Thickening of the colon wall with a marked transparietal inflammatory infiltrate and necrosis (×200). Mean ± SEM are indicated, the number of mice, as well as the statistical significance are indicated.
Figure 6
PPARγ1/− and RXRα1/− mice are more susceptible to TNBS-induced colitis. (A and B) Wallace macroscopic and Ameho histologic inflammation scores of 129/Sv wild-type (WT), PPARγ1/− (A) and RXRα1/− (B) mice 2 d after induction of colitis by TNBS administration. (C) Representative transparietal colon section of 129/Sv wild-type mice (Ameho score 5) 2 d after the induction of colitis by TNBS showing a moderate infiltrate with a necrosis limited to the superficial part of the mucosa (×200). (D and E) Transparietal colon sections in PPARγ1/− (D) and RXRα1/− (E) mice (both Ameho scores of 6) 2 d after the induction of colitis by TNBS. Thickening of the colon wall with a marked transparietal inflammatory infiltrate and necrosis (×200). Mean ± SEM are indicated, the number of mice, as well as the statistical significance are indicated.
Figure 7
TNF-α and IL-1β mRNA levels are reduced by PPARγ and RXR agonists. TNF-α (A) and IL-1β (B) mRNA levels in the colon of mice receiving vehicle only (Control), TNBS, rosiglitazone (Rosi at 20 mg/kg/d), LG101305 (LG at 20 mg/kg/d), or simultaneous administration of rosiglitazone and LG101305 (Rosi + LG both at 1 mg/kg/d) 2 d before the administration of TNBS. Animals were killed 2 d after TNBS administration. Results are expressed as mean ± SEM. mol, molecules. (A): (a) P < 0.001 in control vs. TNBS; (b) P = 0.001 in untreated TNBS colitis vs. mice receiving rosiglitazone; (c) P = 0.076 in untreated TNBS colitis vs. mice receiving LG101305; and (d) P = 0.05 in untreated TNBS colitis vs. mice receiving both rosiglitazone and LG101305. (B): (a) P < 0.001 in control vs. TNBS; (b) P = 0.001 in untreated TNBS colitis vs. mice receiving rosiglitazone; (c) P = 0.003 in untreated TNBS colitis vs. mice receiving LG101305; and (d) P = 0.003 in untreated TNBS colitis vs. mice receiving both rosiglitazone and LG101305.
Figure 7
TNF-α and IL-1β mRNA levels are reduced by PPARγ and RXR agonists. TNF-α (A) and IL-1β (B) mRNA levels in the colon of mice receiving vehicle only (Control), TNBS, rosiglitazone (Rosi at 20 mg/kg/d), LG101305 (LG at 20 mg/kg/d), or simultaneous administration of rosiglitazone and LG101305 (Rosi + LG both at 1 mg/kg/d) 2 d before the administration of TNBS. Animals were killed 2 d after TNBS administration. Results are expressed as mean ± SEM. mol, molecules. (A): (a) P < 0.001 in control vs. TNBS; (b) P = 0.001 in untreated TNBS colitis vs. mice receiving rosiglitazone; (c) P = 0.076 in untreated TNBS colitis vs. mice receiving LG101305; and (d) P = 0.05 in untreated TNBS colitis vs. mice receiving both rosiglitazone and LG101305. (B): (a) P < 0.001 in control vs. TNBS; (b) P = 0.001 in untreated TNBS colitis vs. mice receiving rosiglitazone; (c) P = 0.003 in untreated TNBS colitis vs. mice receiving LG101305; and (d) P = 0.003 in untreated TNBS colitis vs. mice receiving both rosiglitazone and LG101305.
Figure 8
NF-κB, JNK, and p38 kinase activities 2 d after TNBS colitis induction in mice treated preventively with rosiglitazone. (A) NF-κB. Proteins in colon homogenates obtained from three control mice (Control), three mice receiving TNBS (TNBS), and three mice receiving TNBS and rosiglitazone (Rosi, 20 mg/kg/day) were used in electrophoretic mobility shift assay (EMSA) reactions as described in Materials and Methods. The top panel shows autoradiographs of the EMSA assay using a radiolabeled NF-κB consensus binding site. The bottom panel shows a graphical presentation obtained after scanning the corresponding autoradiographs. Compared with the most intense NF-κB complex radiolabeled signal obtained in mice receiving TNBS, control (Cont) corresponding to a competition with an excess of cold NF-κB showed a marked decrease of the radiolabeling. (B and C) JNK (B) and p38 kinase (C) activities in immunoprecipitates of colon extracts obtained from three control mice (Control), three mice receiving TNBS (TNBS), and three mice receiving TNBS and treated preventively with rosiglitazone (Rosi, 20 mg/kg/day). The top panel shows autoradiographs, showing the phosphorylation status of the GST-ATF2 fusion protein used as a substrate to measure the kinase activities in the JNK and p38 immunoprecipitates. The bottom panel shows a graphical presentation obtained after scanning the corresponding autoradiographs. AU, arbitrary units.
Similar articles
- Sensitization of diabetic and obese mice to insulin by retinoid X receptor agonists.
Mukherjee R, Davies PJ, Crombie DL, Bischoff ED, Cesario RM, Jow L, Hamann LG, Boehm MF, Mondon CE, Nadzan AM, Paterniti JR Jr, Heyman RA. Mukherjee R, et al. Nature. 1997 Mar 27;386(6623):407-10. doi: 10.1038/386407a0. Nature. 1997. PMID: 9121558 - Role of peroxisome proliferator-activated receptor gamma and retinoid X receptor heterodimer in hepatogastroenterological diseases.
Dubuquoy L, Dharancy S, Nutten S, Pettersson S, Auwerx J, Desreumaux P. Dubuquoy L, et al. Lancet. 2002 Nov 2;360(9343):1410-8. doi: 10.1016/S0140-6736(02)11395-X. Lancet. 2002. PMID: 12424006 Review. - The pleiotropic functions of peroxisome proliferator-activated receptor gamma.
Debril MB, Renaud JP, Fajas L, Auwerx J. Debril MB, et al. J Mol Med (Berl). 2001;79(1):30-47. doi: 10.1007/s001090000145. J Mol Med (Berl). 2001. PMID: 11327101 Review.
Cited by
- Ulcerative colitis impairs the acylethanolamide-based anti-inflammatory system reversal by 5-aminosalicylic acid and glucocorticoids.
Suárez J, Romero-Zerbo Y, Márquez L, Rivera P, Iglesias M, Bermúdez-Silva FJ, Andreu M, Rodríguez de Fonseca F. Suárez J, et al. PLoS One. 2012;7(5):e37729. doi: 10.1371/journal.pone.0037729. Epub 2012 May 25. PLoS One. 2012. PMID: 22662201 Free PMC article. - Adipokines and the role of visceral adipose tissue in inflammatory bowel disease.
Karrasch T, Schaeffler A. Karrasch T, et al. Ann Gastroenterol. 2016 Oct-Dec;29(4):424-438. doi: 10.20524/aog.2016.0077. Epub 2016 Sep 6. Ann Gastroenterol. 2016. PMID: 27708507 Free PMC article. Review. - Macrophages in synovial inflammation.
Kennedy A, Fearon U, Veale DJ, Godson C. Kennedy A, et al. Front Immunol. 2011 Oct 10;2:52. doi: 10.3389/fimmu.2011.00052. eCollection 2011. Front Immunol. 2011. PMID: 22566842 Free PMC article. - Significance of anti-inflammatory effects of PPARgamma agonists?
Rogler G. Rogler G. Gut. 2006 Aug;55(8):1067-9. doi: 10.1136/gut.2005.089946. Gut. 2006. PMID: 16849341 Free PMC article. Review. - Peroxisome proliferator activated receptor-γ and traumatic brain injury.
Qi L, Jacob A, Wang P, Wu R. Qi L, et al. Int J Clin Exp Med. 2010 Sep 23;3(4):283-92. Int J Clin Exp Med. 2010. PMID: 21072262 Free PMC article.
References
- Schoonjans K., Martin G., Staels B., Auwerx J. Peroxisome proliferator-activated receptors, orphans with ligands and functions. Curr. Opin. Lipidol. 1997;8:159–166. - PubMed
- Desvergne B., Wahli W. Peroxisome proliferator-activated receptorsnuclear control of metabolism. Endocr. Rev. 1999;20:649–688. - PubMed
- Willson T.M., Brown P.J., Sternbach D.D., Henke B.R. The PPARsfrom orphan receptors to drug discovery. J. Med. Chem. 2000;43:527–550. - PubMed
- Schoonjans K., Auwerx J. Thiazolidinedionesan update. Lancet. 2000;355:1008–1010. - PubMed
- Jiang C., Ting A.T., Seed B. PPARγ agonists inhibit production of monocyte inflammatory cytokines. Nature. 1998;391:82–86. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous