GPI anchoring leads to sphingolipid-dependent retention of endocytosed proteins in the recycling endosomal compartment - PubMed (original) (raw)
GPI anchoring leads to sphingolipid-dependent retention of endocytosed proteins in the recycling endosomal compartment
S Chatterjee et al. EMBO J. 2001.
Abstract
Glycosylphosphatidylinositol (GPI) anchoring is important for the function of several proteins in the context of their membrane trafficking pathways. We have shown previously that endocytosed GPI-anchored proteins (GPI-APs) are recycled to the plasma membrane three times more slowly than other membrane components. Recently, we found that GPI-APs are delivered to endocytic organelles, devoid of markers of the clathrin-mediated pathway, prior to their delivery to a common recycling endosomal compartment (REC). Here we show that the rate-limiting step in the recycling of GPI-APs is their slow exit from the REC; replacement of the GPI anchor with a transmembrane protein sequence abolishes retention in this compartment. Depletion of endogenous sphingolipid levels using sphingolipid synthesis inhibitors or in a sphingolipid-synthesis mutant cell line specifically enhances the rate of endocytic recycling of GPI-APs to that of other membrane components. We have shown previously that endocytic retention of GPI-APs is also relieved by cholesterol depletion. These findings strongly suggest that functional retention of GPI-APs in the REC occurs via their association with sphingolipid and cholesterol-enriched sorting platforms or 'rafts'.
Figures
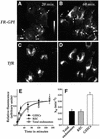
Fig. 1. Trafficking kinetics of FR-GPI measured in endocytic compartments of CHO cells. FRαTb-1 cells were incubated with PLR to determine the extent of accumulation of endocytosed FR-GPI in the peripheral GEECs (arrows) and the pericentriolar REC (arrowheads) after a 20 (A) or 60 min (B) pulse of the label (see Materials and methods). (A) and (B) show gray-scale intensities of endocytosed PLR normalized to surface FR-GPI expression. The bar shows a gradient of gray-scale intensities with the lowest (black) at the bottom of the bar to the highest (white) at the top of the bar. (C and D) REC-localized Cy5-Tf images of the same cells in (A) and (B), obtained as described in Materials and methods. The REC is marked with a white boundary for two representative cells. Scale bar represents 5 µm. (E) Change in surface receptor-normalized fluorescence in intracellular compartments plotted against time of incubation for the total endosomal FR-GPI (open squares), FR-GPI in REC (open triangle) and FR-GPI in GEECs (filled circles). The lines represent the best fit for the data points using the kinetic equation described in Materials and methods. Note that GEECs reach a steady state of PLR labeling much more quickly than the REC or the total endosomal receptors. (F) Rate constant of exit (_k_e) from the indicated compartments.
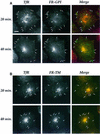
Fig. 2. Retention of FR-GPI in the REC requires the GPI anchor. FRαTb-1 (A) and FR-TMTb-1 (B) cells expressing FR-GPI and FR-TM, respectively, were labeled with PLR and Cy5-Tf for the indicated times and imaged using a wide-field microscope. Pseudo-colored merges of PLR (encoded green) and Cy5-Tf (encoded red) images are shown in the extreme right-hand panel (‘Merge’). Images at the two time points in (A) and (B) were selected for matching total TfR expression and the endosomal PLR fluorescence was normalized to surface receptor expression. Note the change in PLR fluorescence in the REC (arrowheads) compared with that in peripheral endosomes in (A) (arrows indicate GEECs) or (B) (arrows indicate sorting endosomes) between the 20 and 40 min time points. Scale bar represents 10 µm.
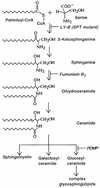
Fig. 3. Schematic of the sphingolipid biosynthesis pathway. The sites of action of the various sphingolipid inhibitors used in this study and the location of the sphingolipid synthesis block in the mutant cell line, LY-B, are indicated by italic text.
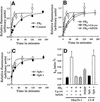
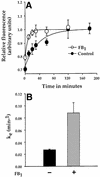
Fig. 4. Sphingolipid depletion increases the rate of recycling of FR-GPI. Fluorescence associated with internalized FR-GPI bound to PLF at indicated times was normalized to surface receptor levels and plotted against time in untreated (control; filled circles) and FB1-treated (open circles) FRαTb-1 cells (A and B) and in FB1-treated FRαTb-1 cells supplemented with exogenous _N_-hexanoyl-
d
-sphingosine (C6-cer; open squares) and _N_-palmitoyl-
dl
-_erythro_-dihydrosphingosine (NPDS; open triangles). Fluorescence associated with internalized PLF bound to FR-GPI at the indicated times was normalized to surface receptor levels and plotted against time in the FR-GPI-expressing LY-B cell line (C) grown in sphingolipid-deficient (Sph–; open circles) or sphingolipid-sufficient medium (Sph+; filled circles). The solid lines in (A)–(C) are the best fit for the data points using the kinetic equation described in Materials and methods. (D) Rate constants of FR-GPI recycling (_k_e) under various treatment conditions. Data are derived from two to six independent experiments for each rate constant determination.
Fig. 5. Rate of recycling of DAF in sphingolipid-depleted cells. (A) DAF-associated fluorescence intensity at the indicated times was normalized to surface receptors and plotted against time in untreated (control; filled circles) and FB1-treated (FB1; open circles) cells. The solid lines represent the best fit for the data points using the kinetic equation described in Materials and methods. (B) Rate constants for DAF recycling in treated and untreated cells.
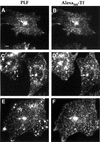
Fig. 6. Endocytic pathway of FR-GPI and TfR in sphingolipid-depleted cells. FRαTb-1 cells were incubated with PLF and Alexa568-Tf to steady state, and images of FR-GPI (A, C and E) and TfR (B, D and F) were collected using a wide-field microscope for untreated (A and B), FB1-treated (C and D) and C6-ceramide-replenished FB1-treated cells (E and F). Note that the majority of the intracellular FR-GPI is extensively co-localized with TfR in the REC (arrows). Scale bar represents 10 µm.
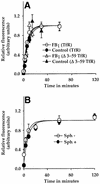
Fig. 7. Sphingolipid depletion does not affect the rate of TfR recycling. (A) Internalized Cy3-Tf fluorescence bound to TfR (circles) or δ3–59TfR (triangles) at indicated times was normalized to surface receptor expression and plotted against time in untreated (control; filled symbols) and FB1-treated (FB1; open symbols) cells, in FRαTb-1 (TfR) and δ3–59TRVb (δ3–59TfR) cells, respectively. (B) Internalized Cy5-Tf fluorescence bound to TfR at the indicated times was normalized to surface receptor expression and plotted against time in LY-B cells grown in sphingolipid-deficient (open circle; Sph–) or sphingolipid-sufficient medium (filled circle; Sph+). The solid lines in (A) and (B) represent the best fit for the data points using the kinetic equation described in Materials and methods.
Similar articles
- Endocytosis of glycosylphosphatidylinositol-anchored proteins.
Lakhan SE, Sabharanjak S, De A. Lakhan SE, et al. J Biomed Sci. 2009 Oct 15;16(1):93. doi: 10.1186/1423-0127-16-93. J Biomed Sci. 2009. PMID: 19832981 Free PMC article. Review. - Assessment of the roles of ordered lipid microdomains in post-endocytic trafficking of glycosyl-phosphatidylinositol-anchored proteins in mammalian fibroblasts.
Refaei M, Leventis R, Silvius JR. Refaei M, et al. Traffic. 2011 Aug;12(8):1012-24. doi: 10.1111/j.1600-0854.2011.01206.x. Epub 2011 Jun 22. Traffic. 2011. PMID: 21696526 - Cholesterol-dependent retention of GPI-anchored proteins in endosomes.
Mayor S, Sabharanjak S, Maxfield FR. Mayor S, et al. EMBO J. 1998 Aug 17;17(16):4626-38. doi: 10.1093/emboj/17.16.4626. EMBO J. 1998. PMID: 9707422 Free PMC article. - Differential sorting and fate of endocytosed GPI-anchored proteins.
Fivaz M, Vilbois F, Thurnheer S, Pasquali C, Abrami L, Bickel PE, Parton RG, van der Goot FG. Fivaz M, et al. EMBO J. 2002 Aug 1;21(15):3989-4000. doi: 10.1093/emboj/cdf398. EMBO J. 2002. PMID: 12145200 Free PMC article. - Glycosylphosphatidylinositol-anchored proteins: Membrane organization and transport.
Zurzolo C, Simons K. Zurzolo C, et al. Biochim Biophys Acta. 2016 Apr;1858(4):632-9. doi: 10.1016/j.bbamem.2015.12.018. Epub 2015 Dec 17. Biochim Biophys Acta. 2016. PMID: 26706096 Review.
Cited by
- Cholesterol level regulates endosome motility via Rab proteins.
Chen H, Yang J, Low PS, Cheng JX. Chen H, et al. Biophys J. 2008 Feb 15;94(4):1508-20. doi: 10.1529/biophysj.106.099366. Epub 2007 Nov 2. Biophys J. 2008. PMID: 17981910 Free PMC article. - The coxsackie B virus and adenovirus receptor resides in a distinct membrane microdomain.
Ashbourne Excoffon KJ, Moninger T, Zabner J. Ashbourne Excoffon KJ, et al. J Virol. 2003 Feb;77(4):2559-67. doi: 10.1128/jvi.77.4.2559-2567.2003. J Virol. 2003. PMID: 12551994 Free PMC article. - PGAP2 is essential for correct processing and stable expression of GPI-anchored proteins.
Tashima Y, Taguchi R, Murata C, Ashida H, Kinoshita T, Maeda Y. Tashima Y, et al. Mol Biol Cell. 2006 Mar;17(3):1410-20. doi: 10.1091/mbc.e05-11-1005. Epub 2006 Jan 11. Mol Biol Cell. 2006. PMID: 16407401 Free PMC article. - Nanoclusters of GPI-anchored proteins are formed by cortical actin-driven activity.
Goswami D, Gowrishankar K, Bilgrami S, Ghosh S, Raghupathy R, Chadda R, Vishwakarma R, Rao M, Mayor S. Goswami D, et al. Cell. 2008 Dec 12;135(6):1085-97. doi: 10.1016/j.cell.2008.11.032. Cell. 2008. PMID: 19070578 Free PMC article. - Endocytosis of glycosylphosphatidylinositol-anchored proteins.
Lakhan SE, Sabharanjak S, De A. Lakhan SE, et al. J Biomed Sci. 2009 Oct 15;16(1):93. doi: 10.1186/1423-0127-16-93. J Biomed Sci. 2009. PMID: 19832981 Free PMC article. Review.
References
- Ariga T., Macala,L.J., Saito,M., Margolis,R.K., Greene,L.A., Margolis,R.U. and Yu,R.K. (1988) Lipid composition of PC12 pheochromocytoma cells: characterization of globoside as a major neutral glycolipid. Biochemistry, 27, 52–58. - PubMed
- Bamezai A., Goldmacher,V.S. and Rock,K.L. (1992) Internalization of glycosyl-phosphatidylinositol (GPI)-anchored lymphocyte proteins. II. GPI-anchored and transmembrane molecules internalize through distinct pathways. Eur. J. Immunol., 22, 15–21. - PubMed
- Bielawska A., Crane,H.M., Liotta,D., Obeid,L.M. and Hannun,Y.A. (1993) Selectivity of ceramide-mediated biology. Lack of activity of erythro-dihydroceramide. J. Biol. Chem., 268, 26226–26232. - PubMed
- Brown D.A. and London,E. (1998a) Functions of lipid rafts in biological membranes. Annu. Rev. Cell. Dev. Biol., 14, 111–136. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous