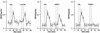Regulated secretion of neurotrophins by metabotropic glutamate group I (mGluRI) and Trk receptor activation is mediated via phospholipase C signalling pathways - PubMed (original) (raw)
Regulated secretion of neurotrophins by metabotropic glutamate group I (mGluRI) and Trk receptor activation is mediated via phospholipase C signalling pathways
M Canossa et al. EMBO J. 2001.
Abstract
Neurotrophins (NTs) play an essential role in modulating activity-dependent neuronal plasticity. In this context, the site and extent of NT secretion are of crucial importance. Here, we demonstrate that the activation of phospolipase C (PLC) and the subsequent mobilization of Ca(2+) from intracellular stores are essential for NT secretion initiated by both Trk and glutamate receptor activation. Mutational analysis of tyrosine residues, highly conserved in the cytoplasmic domain of all Trk receptors, revealed that the activation of PLC-gamma in cultured hippocampal neurons and nnr5 cells is necessary to mobilize Ca(2+) from intracellular stores, the key mechanism for regulated NT secretion. A similar signalling mechanism has been identified for glutamate-mediated NT secretion-which in part depends on the activation of PLC via metabotropic receptors-leading to the mobilization of Ca(2+) from internal stores by inositol trisphosphate. Thus, PLC-mediated signal transduction pathways are the common mechanisms for both Trk- and mGluRI-mediated NT secretion.
Figures
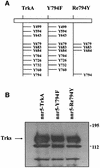
Fig. 1. Stable expression of the wild-type TrkA and receptor mutants in nnr5 cells. (A) Schematic representation of the cytosolic domain of the wild-type TrkA receptor and selected receptor mutants. Y794F represents a TrkA receptor in which the PLC-γ-binding site was inactivated by substituting the tyrosine residue Y794 with a phenyl alanine, while in Re794Y all tyrosine residues have been replaced by phenylalanines, with the exception of Y794. Tyrosine residues Y679, Y683 and Y684 are essential for receptor autophosphorylation and were maintained in all mutants. (B) Western blot of wild-type (nnr5-TrkA) and mutated TrkA receptors (nnr5-Y794F and nnr5-Re794Y) stably expressed in nnr5 cells. Wild-type receptor and mutants were assayed for their levels of expression. We selected clones that, by probing with an anti-pan-Trk antibody, showed comparable levels of receptor protein.
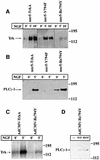
Fig. 2. Comparison between receptor tyrosine and PLC-γ phosphoryl ation in wild-type and mutated TrkA receptors in stably transfected nnr5 cells and adenovirally transduced hippocampal neurons. (A) NGF-mediated tyrosine phosphorylation in nnr5-TrkA, nnr5-Y794F and nnr5-Re794Y clones. Each clone was stimulated for 0, 5 or 10 min with 100 ng/ml NGF and assayed for receptor tyrosine phosphorylation. Wild-type and mutated receptors were immunoprecipitated from cell lysates by an anti-pan-Trk antibody and the samples separated on an SDS–polyacrylamide gel. Western blots were probed with an anti-phosphotyrosine antibody. NGF administered for 5 or 10 min produced a marked tyrosine phosphorylation in wild-type and receptor mutants. (B) NGF-mediated PLC-γ tyrosine phosphorylation in nnr5 clones. The individual clones were stimulated with 100 ng/ml NGF for 0 or 5 min. The cell lysates were immunoprecipitated by agarose-linked anti-phosphotyrosine antibodies and subjected to western blot analysis with a specific anti-PLC-γ antibody. NGF treatment for 5 min was sufficient to induce PLC-γ phosphorylation in nnr5-TrkA and nnr5-Re794Y clones, whereas nnr5-Y794F clones did not show a PLC-γ phosphorylation signal in either the presence or absence of NGF. (C) NGF-mediated tyrosine phosphorylation in cultured hippocampal neurons transduced with TrkA and Re794Y receptors. Transduced neurons were stimulated for 0 and 5 min with 100 ng/ml NGF and assayed for receptor tyrosine phosphorylation. The western blot was performed as in (A). NGF produced a marked tyrosine phosphorylation in the wild-type receptor and mutant. (D) NGF- and BDNF-mediated PLC-γ tyrosine phosphorylation in cultured hippocampal neurons transduced with Re794Y. Cultured hippocampal neurons infected with the adenovirus coding for the Re794Y were stimulated for 0 or 5 min with 100 ng/ml either NGF or BDNF. Lysates of hippocampal neurons were immunoprecipitated by an agarose-conjugated anti-phosphotyrosine antibody. PLC-γ phosphorylation was identified by western blotting as described in (A). Both NGF and BDNF induced PLC-γ tyrosine phosphorylation to a similar extent.
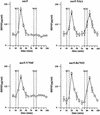
Fig. 3. High potassium- and NGF-mediated BDNF secretion from nnr5-TrkA, nnr5-Y794F and nnr5-Re794Y. Cells were perfused at a flow rate of 0.1 ml/min. Fractions were collected every 5 min. After collecting two fractions, a first stimulation by 50 mM KCl was initiated and four additional fractions collected. After a recovery of 30 min, cells were stimulated by 100 ng/ml NGF. BDNF concentrations were measured by two-site ELISA. Increased BDNF concentrations (expressed in pg/ml) occurred immediately after stimulation. The peak concentration occurred in the following fraction. The 5 min delay between the onset of stimulation and the peak of BDNF secretion is due to the dead volume of the perfusion system. NGF induced BDNF secretion in nnr5-TrkA and nnr5-Re794Y cells, but not in nnr5 or nnr5-Y794F cells. The values given represent the mean ± SE (n = 6).
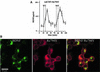
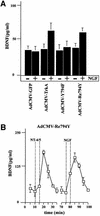
Fig. 4. Adenovirally transduced nnr5 cells. (A) Time course of BDNF secretion from nnr5 cells double transduced with AdCMV-Re794Y and AdCMV-BDNF. Perfusion was performed as described in Figure 3. Two subsequent stimulations by 50 mM KCl and 100 ng/ml NGF were separated by a recovery period of 30 min. Fractions were collected every 5 min and the BDNF concentrations were determined by ELISA. The values presented are the mean ± SE (n = 4). (B) Expression of BDNF and Re794Y mutant in nnr5 cells co-transduced with AdCMV-BDNF and AdCMV-Re794Y. The expression of both BDNF and Re794Y was detected by probing with the hybridoma supernatant (9E10), which recognizes the myc epitope added to the C-terminal domain of BDNF, and with the anti-pan-Trk antibody, which recognizes the C-terminus of Re794Y. Cells were analysed by confocal microscopy. The pictures represent a single confocal scan along a given x–y plane. The BDNFmyc-infected nnr5 cells showed a discontinuous staining pattern characteristic for the localization of BDNF in ER and Golgi compartments (green). The staining obtained with an anti-pan-Trk antibody was localized predominantly at the plasma membrane (red).
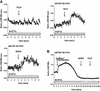
Fig. 5. BDNF secretion from cultured hippocampal neurons expressing TrkA, Y794F and Re794Y receptors. (A) Hippocampal neurons transduced with AdCMV-TrkA, AdCMV-Y794F and AdCMV-Re794Y were stimulated with 100 ng/ml NGF for 10 min under ‘static’ conditions. NGF-induced BDNF secretion occurred only in neurons expressing wild-type TrkA and the Re794Y receptors, but not Y794F or the green fluorescence protein (GFP) taken as a control of virus infection. The values given are the mean ± SE (n = 10). (B) Time course of BDNF secretion induced by NT4/5 via endogenous TrkB or by NGF via transuced Re794Y. Cells were perfused and fractions were collected before, during and after stimulation with 100 ng/ml NT4/5. A second stimulation was initiated by the administration of 100 ng/ml NGF. NT4/5 activated endogenous TrkB receptors, and NGF the TrkA receptor mutant Re794Y. The values given are the mean ± SE (n = 6).
Fig. 6. Changes of intracellular Ca2+ concentrations in hippocampal neurons expressing the Re794Y receptors. (A) Non-transduced hippocampal neurons (controls) and those transduced by Re794Y were loaded with Fura-2/AM. Changes in intracellular Ca2+ concentrations were determined by the ratio of the fluorescence at excitation wavelengths of 340 and 380 nm. In the absence of extracellular Ca2+ (BAPTA), hippocampal neurons expressing the TrkA receptor mutant showed an NGF-mediated increase in [Ca2+]i that could not be distinguished from that obtained by BDNF acting via endogenous TrkB receptors. This is a representative example of eight independent experiments. (B) Effect of the depletion of the intracellular Ca2+ stores by thapsigargine and caffeine on the subsequent Ca2+ signalling by BDNF or NGF. In the absence of extracellular Ca2+ (BAPTA), caffeine/thapsigargine initiated a strong, prolonged Ca2+ signal resulting from the depletion of the Ca2+ stores. Subsequent administration of BDNF and NGF did not elicit a detectable Ca2+ signal, in distinct contrast to the hippocampal neurons not treated with caffeine and thapsigargine. This is a representative example of three independent experiments.
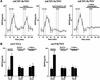
Fig. 7. Dependence on intact Ca2+ stores of NT-mediated NT secretion in hippocampal neurons. (A) BDNF secretion from hippocampal neurons in the absence of extracellular Ca2+ (BAPTA), chelation of intracellular calcium by BAPTA-AM and after depletion of the intracellular Ca2+ stores by caffeine and thapsigargine. Cultured hippocampal neurons co-infected with AdCMV-BDNF and AdCMV-Re794Y were perfused as described in Figure 3. After stimulation with 100 ng/ml NT4/5 for 5 min in normal Ca2+, a second stimulation with 100 ng/ml NGF was performed in Ca2+-free medium or medium containing the Ca2+ chelator BAPTA-AM (10 µM). Removal of extracellular Ca2+ (BAPTA) did not affect the ability of NGF to induce BDNF secretion. However, the effect of NGF on BDNF secretion was abolished by pre-treating hippocampal neurons for 30 min with the cell-permeable BAPTA-AM. Similarly, the depletion of intracellular Ca2+ stores with a combination of caffeine (3 mM) and thapsigargine (10 µM) abolished the NGF-mediated BDNF secretion. The values given represent the mean ± SE (n = 6). (B) Role of Ca2+ in BDNF secretion from nnr5-TrkA or nnr5-Re794Y clones. Similarly to the effects shown in hippocampal neurons, NGF induced BDNF secretion from nnr5 clones in the absence of extracellular Ca2+ (BAPTA). In contrast, chelating the intracellular Ca2+ with BAPTA-AM or depleting intracellular Ca2+ stores with caffeine/thapsigargine prevented NGF-mediated secretion of BDNF. The values given represent the mean ± SE (n = 7).
Fig. 8. Time course of BDNF secretion in native, non-transduced hippocampal slices. Acute hippocampal slices were perfused and fractions were collected over consecutive 5 min periods. BDNF concentrations were determined by ELISA. After a recovery period of 30 min, an initial stimulation by 50 µM glutamate was followed by a second stimulation with 100 µM t-ACPD, AMPA or NMDA. The values given are the mean ± SE (n = 4).
Fig. 9. Analysis of the signal transduction pathway(s) leading to BDNF secretion by glutamate. (A) BDNF secretion initiated by glutamate receptor agonists in cultured hippocampal neurons. Primary cultures of hippocampal neurons were infected with AdCMV-BDNF for 36–48 h. After an equilibration time of 60 min, basal levels of secreted BDNF were determined in the medium collected over a 10 min period under ‘static’ conditions. Stimulation of neurons for 10 min with glutamate (50 µM), AMPA (100 µM) or t-ACPD (100 µM) resulted in increased concentrations of BDNF in the incubation medium. NMDA had no effect. (B) Hippocampal neurons, pre-treated with the specific antagonists of AMPA and mGluRI receptors, CNQX (50 µM) and AIDA (500 µM), respectively, were tested for the effects of AMPA and t-ACPD. (C) Influence of Ca2+ stores on AMPA- and t-ACPD-mediated BDNF secretion. In hippocampal neurons, treatment with thapsigargine (10 µM) and caffeine (3 mM) initiated BDNF secretion but abolished the subsequent secretion of BDNF induced by AMPA and t-ACPD. The values given represent the mean ± SE (n = 6).
Similar articles
- NMDA-receptor regulation of muscarinic-receptor stimulated inositol 1,4,5-trisphosphate production and protein kinase C activation in single cerebellar granule neurons.
Young KW, Garro MA, Challiss RA, Nahorski SR. Young KW, et al. J Neurochem. 2004 Jun;89(6):1537-46. doi: 10.1111/j.1471-4159.2004.02458.x. J Neurochem. 2004. PMID: 15189357 - Promoting neurotrophic effects by GPCR ligands.
Jeanneteau F, Chao MV. Jeanneteau F, et al. Novartis Found Symp. 2006;276:181-9; discussion 189-92, 233-7, 275-81. Novartis Found Symp. 2006. PMID: 16805430 Review. - Insights on the Functional Interaction between Group 1 Metabotropic Glutamate Receptors (mGluRI) and ErbB Receptors.
Ledonne A, Mercuri NB. Ledonne A, et al. Int J Mol Sci. 2020 Oct 24;21(21):7913. doi: 10.3390/ijms21217913. Int J Mol Sci. 2020. PMID: 33114459 Free PMC article. Review.
Cited by
- Neurobiology of local and intercellular BDNF signaling.
Sasi M, Vignoli B, Canossa M, Blum R. Sasi M, et al. Pflugers Arch. 2017 Jun;469(5-6):593-610. doi: 10.1007/s00424-017-1964-4. Epub 2017 Mar 9. Pflugers Arch. 2017. PMID: 28280960 Free PMC article. Review. - Neuronal Scaffold Protein ARMS Interacts with Synaptotagmin-4 C2AB through the Ankyrin Repeat Domain with an Unexpected Mode.
Zhang F, Chen J, Li Y, Ye J, Wang C. Zhang F, et al. Int J Mol Sci. 2023 Nov 30;24(23):16993. doi: 10.3390/ijms242316993. Int J Mol Sci. 2023. PMID: 38069318 Free PMC article. - Role for Endogenous BDNF in Endocannabinoid-Mediated Long-Term Depression at Neocortical Inhibitory Synapses.
Zhao L, Yeh ML, Levine ES. Zhao L, et al. eNeuro. 2015 Feb 28;2(2):ENEURO.0029-14.2015. doi: 10.1523/ENEURO.0029-14.2015. eNeuro. 2015. PMID: 25938134 Free PMC article. - Exercise can rescue recognition memory impairment in a model with reduced adult hippocampal neurogenesis.
Lafenêtre P, Leske O, Ma-Högemeie Z, Haghikia A, Bichler Z, Wahle P, Heumann R. Lafenêtre P, et al. Front Behav Neurosci. 2010 Feb 22;3:34. doi: 10.3389/neuro.08.034.2009. eCollection 2010. Front Behav Neurosci. 2010. PMID: 20204139 Free PMC article. - Hippocampal neurons recycle BDNF for activity-dependent secretion and LTP maintenance.
Santi S, Cappello S, Riccio M, Bergami M, Aicardi G, Schenk U, Matteoli M, Canossa M. Santi S, et al. EMBO J. 2006 Sep 20;25(18):4372-80. doi: 10.1038/sj.emboj.7601303. Epub 2006 Sep 7. EMBO J. 2006. PMID: 16957779 Free PMC article.
References
- Aibel L., Martinzanca,D., Perez,P. and Chao,M.V. (1998) Functional expression of TrkA receptors in hippocampal neurons. J. Neurosci. Res., 54, 424–431. - PubMed
- Berridge M.J. (1998) Neuronal calcium signaling. Neuron, 21, 13–26. - PubMed
- Blöchl A. and Sirrenberg,C. (1996) Neurotrophins stimulate the release of dopamine from rat mesencephalic neurons via Trk and p75Lntr receptors. J. Biol. Chem., 271, 21100–21107. - PubMed
- Blöchl A. and Thoenen,H. (1995) Characterization of nerve growth factor (NGF) release from hippocampal neurons: evidence for a constitutive and an unconventional sodium-dependent regulated pathway. Eur. J. Neurosci., 7, 1220–1228. - PubMed
- Blöchl A. and Thoenen,H. (1996) Localization of cellular storage compartments and sites of constitutive and activity-dependent release of nerve growth factor (NGF) in primary cultures of hippocampal neurons. Mol. Cell. Neurosci., 7, 173–190. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous