Nuclear import of histone H2A and H2B is mediated by a network of karyopherins - PubMed (original) (raw)
Nuclear import of histone H2A and H2B is mediated by a network of karyopherins
N Mosammaparast et al. J Cell Biol. 2001.
Abstract
The first step in the assembly of new chromatin is the cell cycle-regulated synthesis and nuclear import of core histones. The core histones include H2A and H2B, which are assembled into nucleosomes as heterodimers. We show here that the import of histone H2A and H2B is mediated by several members of the karyopherin (Kap; importin) family. An abundant complex of H2A, H2B, and Kap114p was detected in cytosol. In addition, two other Kaps, Kap121p and Kap123p, and the histone chaperone Nap1p were isolated with H2A and H2B. Nap1p is not necessary for the formation of the Kap114p-H2A/H2B complex or for import of H2A and H2B. We demonstrate that both histones contain a nuclear localization sequence (NLS) in the amino-terminal tail. Fusions of the NLSs to green fluorescent protein were specifically mislocalized to the cytoplasm in kap mutant strains. In addition, we detected a specific mislocalization in a kap95 temperature-sensitive strain, suggesting that this Kap is also involved in the import of H2A and H2B in vivo. Importantly, we show that Kap114p, Kap121p, and Kap95 interact directly with both histone NLSs and that RanGTP inhibits this association. These data suggest that the import of H2A and H2B is mediated by a network of Kaps, in which Kap114p may play the major role.
Figures
Figure 1
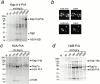
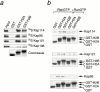
Kap114 binds to histones H2A and H2B in the cytoplasm. (a) Kap114-PrA and associated proteins were isolated from cytosol by IgG-Sepharose, eluted with a MgCl2 gradient, separated by SDS-PAGE, and visualized by Coomassie blue staining. The bands representing Kap114-PrA, TBP and histones H2A and H2B are indicated. (b) Cells expressing either H2A-PrA or H2B-PrA were fixed and the PrA moiety detected by indirect immunofluorescence. The coincident DAPI staining is shown. (c) H2A-PrA and associated proteins were isolated from cytosol. Bands representing Kap114p, Nap1p, H2A-PrA, and H2B are indicated. (d) H2B-PrA and associated proteins were isolated as described. Bands representing Kap114p, Nap1p, and H2B-PrA are indicated. Positions of molecular mass standards in kilodaltons are shown.
Figure 3
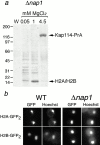
Nap1p is not required for the formation of a functional Kap114p-H2A/H2B complex. (a) Kap114-PrA was expressed in a Δ_nap1_ strain. Kap114-PrA and associated proteins were isolated as described and bands representing Kap114-PrA and H2A/H2B are indicated. Positions of molecular mass standards in kilodaltons are shown. (b) H2A-GFP2 or H2B-GFP2 were expressed in wild-type (WT) and Δ_nap1_ strains and detected by fluorescence imaging. The coincident Hoechst staining is shown.
Figure 2
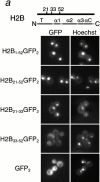
Histone H2A and H2B both contain distinct NLSs. Different fragments of H2B (a) and H2A (b) (as indicated by amino acid number) were expressed as GFP2 fusions in wild-type cells, and the GFP moiety detected by fluorescence imaging. The coincident Hoechst staining is shown. Schematic of H2A and H2B drawn to scale; T, amino-terminal tail; α, alpha helix; αC, carboxy-terminal alpha-helix; CT, carboxy-terminal tail.
Figure 2
Histone H2A and H2B both contain distinct NLSs. Different fragments of H2B (a) and H2A (b) (as indicated by amino acid number) were expressed as GFP2 fusions in wild-type cells, and the GFP moiety detected by fluorescence imaging. The coincident Hoechst staining is shown. Schematic of H2A and H2B drawn to scale; T, amino-terminal tail; α, alpha helix; αC, carboxy-terminal alpha-helix; CT, carboxy-terminal tail.
Figure 4
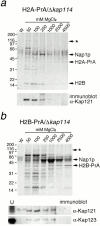
H2A and H2B interact with additional Kaps in the absence of Kap114p. (a) H2A-PrA was expressed in a Δ_kap114_ strain. H2A-PrA and associated proteins were isolated as described. Bands representing potential Kaps (*), Nap1p, H2A-PrA, and H2B are indicated. Identical fractions were Western blotted with an antibody to Kap121p, shown below. (b) H2B-PrA was expressed in a Δ_kap114_ strain. H2B-PrA and associated proteins were isolated as described. Bands representing potential Kaps (*), Nap1p, and H2B-PrA are indicated. The fractions were Western blotted with antibodies to Kap121p and Kap123p as indicated below. (U) Unbound supernatant fraction. Positions of molecular mass standards in kilodaltons are shown.
Figure 5
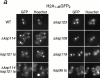
Kap114p, Kap121p, Kap123p, and Kap95p participate in the nuclear import of H2A and H2B. H2A1-46GFP2 (a) or H2B1-52GFP2 (b) were expressed in wild-type (WT) and kap mutant strains (as indicated) and the GFP moiety detected by fluorescence imaging. The coincident Hoechst staining is shown. Strains were grown at 30°C, except for strains with temperature-sensitive alleles (kap121ts and kap95ts), which were grown at room temperature. Images were identically manipulated in Photoshop. (c) Images of the NLS reporter-bearing strains, grown as above or as indicated, were captured by fluorescence imaging. The mean fluorescence intensity of a defined pixel area was measured in the nucleus (N) and cytoplasm (C), and used to calculate the N:C ratio of mean fluorescence intensity. The mean ratio for 50 cells is shown (columns), as well as the SD.
Figure 5
Kap114p, Kap121p, Kap123p, and Kap95p participate in the nuclear import of H2A and H2B. H2A1-46GFP2 (a) or H2B1-52GFP2 (b) were expressed in wild-type (WT) and kap mutant strains (as indicated) and the GFP moiety detected by fluorescence imaging. The coincident Hoechst staining is shown. Strains were grown at 30°C, except for strains with temperature-sensitive alleles (kap121ts and kap95ts), which were grown at room temperature. Images were identically manipulated in Photoshop. (c) Images of the NLS reporter-bearing strains, grown as above or as indicated, were captured by fluorescence imaging. The mean fluorescence intensity of a defined pixel area was measured in the nucleus (N) and cytoplasm (C), and used to calculate the N:C ratio of mean fluorescence intensity. The mean ratio for 50 cells is shown (columns), as well as the SD.
Figure 5
Kap114p, Kap121p, Kap123p, and Kap95p participate in the nuclear import of H2A and H2B. H2A1-46GFP2 (a) or H2B1-52GFP2 (b) were expressed in wild-type (WT) and kap mutant strains (as indicated) and the GFP moiety detected by fluorescence imaging. The coincident Hoechst staining is shown. Strains were grown at 30°C, except for strains with temperature-sensitive alleles (kap121ts and kap95ts), which were grown at room temperature. Images were identically manipulated in Photoshop. (c) Images of the NLS reporter-bearing strains, grown as above or as indicated, were captured by fluorescence imaging. The mean fluorescence intensity of a defined pixel area was measured in the nucleus (N) and cytoplasm (C), and used to calculate the N:C ratio of mean fluorescence intensity. The mean ratio for 50 cells is shown (columns), as well as the SD.
Figure 6
Kap114p, Kap121p, and Kap95p bind directly to the H2A and H2B NLSs. (a) Reticulocyte lysate containing 35S-methionine–labeled Kap114p, Kap95p, Kap121p, or Kap108p was incubated with Sepharose-bound GST (GST), GST-H2A amino acids 1–46 (GST-H2A), or GST-H2B amino acids 1–52 (GST-H2B). The bound material was separated by SDS-PAGE. Kaps were visualized by fluorography of the gels (top four panels). The reticulocyte lysate input (2% of reaction) is also shown. The GST and GST-fusion proteins for a representative experiment, visualized by Coomassie blue staining, are shown (bottom). (b) 1 μg of purified, bacterially expressed Kap114p, Kap121p, or Kap95p was incubated with Sepharose-bound GST (GST), GST-H2A amino acids 1–46 (GST-H2A), or GST-H2B amino acids 1–52 (GST-H2B). Where indicated, the Kap was preincubated with 20 μM human RanGTP Q69L. The bound material was separated by SDS-PAGE and visualized by Coomassie blue staining. Positions of GST-fusion proteins are indicated; additional lower bands are probably degradation products.
Similar articles
- A role for nucleosome assembly protein 1 in the nuclear transport of histones H2A and H2B.
Mosammaparast N, Ewart CS, Pemberton LF. Mosammaparast N, et al. EMBO J. 2002 Dec 2;21(23):6527-38. doi: 10.1093/emboj/cdf647. EMBO J. 2002. PMID: 12456659 Free PMC article. - Modulation of histone deposition by the karyopherin kap114.
Mosammaparast N, Del Rosario BC, Pemberton LF. Mosammaparast N, et al. Mol Cell Biol. 2005 Mar;25(5):1764-78. doi: 10.1128/MCB.25.5.1764-1778.2005. Mol Cell Biol. 2005. PMID: 15713633 Free PMC article. - Nuclear import of TFIIB is mediated by Kap114p, a karyopherin with multiple cargo-binding domains.
Hodges JL, Leslie JH, Mosammaparast N, Guo Y, Shabanowitz J, Hunt DF, Pemberton LF. Hodges JL, et al. Mol Biol Cell. 2005 Jul;16(7):3200-10. doi: 10.1091/mbc.e04-11-0990. Epub 2005 May 11. Mol Biol Cell. 2005. PMID: 15888545 Free PMC article. - Nuclear import of histones.
Bernardes NE, Chook YM. Bernardes NE, et al. Biochem Soc Trans. 2020 Dec 18;48(6):2753-2767. doi: 10.1042/BST20200572. Biochem Soc Trans. 2020. PMID: 33300986 Free PMC article. Review. - Histone chaperones link histone nuclear import and chromatin assembly.
Keck KM, Pemberton LF. Keck KM, et al. Biochim Biophys Acta. 2013 Mar-Apr;1819(3-4):277-89. Biochim Biophys Acta. 2013. PMID: 24459730 Review.
Cited by
- Histone H2B-IFI16 Recognition of Nuclear Herpesviral Genome Induces Cytoplasmic Interferon-β Responses.
Iqbal J, Ansari MA, Kumar B, Dutta D, Roy A, Chikoti L, Pisano G, Dutta S, Vahedi S, Veettil MV, Chandran B. Iqbal J, et al. PLoS Pathog. 2016 Oct 20;12(10):e1005967. doi: 10.1371/journal.ppat.1005967. eCollection 2016 Oct. PLoS Pathog. 2016. PMID: 27764250 Free PMC article. - Multiple pathways contribute to nuclear import of core histones.
Mühlhäusser P, Müller EC, Otto A, Kutay U. Mühlhäusser P, et al. EMBO Rep. 2001 Aug;2(8):690-6. doi: 10.1093/embo-reports/kve168. EMBO Rep. 2001. PMID: 11493596 Free PMC article. - Molecular basis of RanGTP-activated nucleosome assembly with Histones H2A-H2B bound to Importin-9.
Shaffer JM, Jiou J, Tripathi K, Olaluwoye OS, Fung HYJ, Chook YM, D'Arcy S. Shaffer JM, et al. bioRxiv [Preprint]. 2023 Jan 28:2023.01.27.525896. doi: 10.1101/2023.01.27.525896. bioRxiv. 2023. PMID: 36747879 Free PMC article. Updated. Preprint. - N-terminal alpha-methylation of RCC1 is necessary for stable chromatin association and normal mitosis.
Chen T, Muratore TL, Schaner-Tooley CE, Shabanowitz J, Hunt DF, Macara IG. Chen T, et al. Nat Cell Biol. 2007 May;9(5):596-603. doi: 10.1038/ncb1572. Epub 2007 Apr 15. Nat Cell Biol. 2007. PMID: 17435751 Free PMC article. - Kap121p-mediated nuclear import is required for mating and cellular differentiation in yeast.
Leslie DM, Grill B, Rout MP, Wozniak RW, Aitchison JD. Leslie DM, et al. Mol Cell Biol. 2002 Apr;22(8):2544-55. doi: 10.1128/MCB.22.8.2544-2555.2002. Mol Cell Biol. 2002. PMID: 11909949 Free PMC article.
References
- Adams C.R., Kamakaka R.T. Chromatin assemblybiochemical identities and genetic redundancy. Curr. Opin. Genet. Dev. 1999;9:185–190. - PubMed
- Aitchison J.D., Blobel G., Rout M.P. Kap104pa karyopherin involved in the nuclear transport of messenger RNA binding proteins. Science. 1996;274:624–627. - PubMed
- Aitchison J.D., Rout M.P., Marelli M., Blobel G., Wozniak R.W. Two novel related yeast nucleoporins Nup170p and Nup157pcomplementation with the vertebrate homologue Nup155p and functional interactions with the yeast nuclear pore-membrane protein Pom152p. J. Cell Biol. 1995;131:1133–1148. - PMC - PubMed
- Breeuwer M., Goldfarb D.S. Facilitated nuclear transport of histone H1 and other small nucleophilic proteins. Cell. 1990;60:999–1008. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous