Selective binding of perfringolysin O derivative to cholesterol-rich membrane microdomains (rafts) - PubMed (original) (raw)
Selective binding of perfringolysin O derivative to cholesterol-rich membrane microdomains (rafts)
A A Waheed et al. Proc Natl Acad Sci U S A. 2001.
Abstract
There is increasing evidence that sphingolipid- and cholesterol-rich microdomains (rafts) exist in the plasma membrane. Specific proteins assemble in these membrane domains and play a role in signal transduction and many other cellular events. Cholesterol depletion causes disassembly of the raft-associated proteins, suggesting an essential role of cholesterol in the structural maintenance and function of rafts. However, no tool has been available for the detection and monitoring of raft cholesterol in living cells. Here we show that a protease-nicked and biotinylated derivative (BCtheta) of perfringolysin O (theta-toxin) binds selectively to cholesterol-rich microdomains of intact cells, the domains that fulfill the criteria of rafts. We fractionated the homogenates of nontreated and Triton X-100-treated platelets after incubation with BCtheta on a sucrose gradient. BCtheta was predominantly localized in the floating low-density fractions (FLDF) where cholesterol, sphingomyelin, and Src family kinases are enriched. Immunoelectron microscopy demonstrated that BCtheta binds to a subpopulation of vesicles in FLDF. Depletion of 35% cholesterol from platelets with cyclodextrin, which accompanied 76% reduction in cholesterol from FLDF, almost completely abolished BCtheta binding to FLDF. The staining patterns of BCtheta and filipin in human epidermoid carcinoma A431 cells with and without cholesterol depletion suggest that BCtheta binds to specific membrane domains on the cell surface, whereas filipin binding is indiscriminate to cell cholesterol. Furthermore, BCtheta binding does not cause any damage to cell membranes, indicating that BCtheta is a useful probe for the detection of membrane rafts in living cells.
Figures
Figure 1
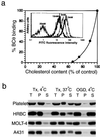
Characteristics of BCθ binding to intact cells. (a) BCθ binding depends on cholesterol contents in cell membranes. Cholesterol was depleted from platelets with 5, 10, and 20 mM 2OHpβCD. The amount of BCθ bound to the platelets was quantified by flow-cytometric analysis (Inset) using fluorescein–avidin and plotted against cholesterol content. The values are expressed as percentage of nontreated control. Cholesterol in untreated platelets was 33 mol% of total lipids. (b) BCθ bound to cells was retained in the Tx-insoluble fraction. After incubation with BCθ, platelets, erythrocytes, MOLT-4, and A431 cells were extracted with 1% Tx (4°C or 37°C) or 60 mM octyl glucoside (OGD) (4°C) and the insoluble fraction was separated by centrifugation at 15,000 × g. The distribution of BCθ in insoluble pellet and soluble fractions were determined. T, total; P, pellet; S, soluble fractions.
Figure 2
BCθ is enriched in FLDF. BCθ-bound platelets were treated with (+Tx) or without Tx (-Tx), sonicated, subjected to a sucrose gradient centrifugation, and fractionated from the top (fractions 1–11 and pellet, fraction 12). The distribution of BCθ (a, +Tx and -Tx), cholesterol (c, open bars, +Tx and -Tx), and total protein (c, closed circles, +Tx and -Tx) in the gradient fractions was analyzed. In parallel experiments the distribution of sphingomyelin (SM) (b, +Tx and -Tx) and tyrosine-kinases (e, +Tx) was determined. (d) The Tx-insoluble pellet obtained at 4°C by centrifugation at 15,000 × g was subjected to density gradient fractionation and the cholesterol content in each fraction was determined. T, total platelets. On BCθ binding a shift in the distribution patterns of lipids and proteins was observed from fractions 2–4 to fractions 3–5 (+Tx). Because Tx in bottom fractions (–12) interferes with the separation of lipids on TLC plates, the distribution pattern of sphingomyelin in these fractions was not shown (b, +Tx).
Figure 3
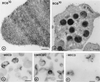
IEM detection of cholesterol on erythrocytes, resting platelets, and isolated low-density membranes. (a and b) IEM detection of cell surface cholesterol of erythrocytes (a) and platelets (b). Washed cells were incubated with BCθ. Ultrathin cryosections were immunolabeled with anti-biotin and 10 nm protein-A gold. (c and_d_) Low-density membranes isolated from platelets by sucrose gradient centrifugation after BCθ binding. BCθ was immunolabeled with anti-biotin and 10 nm protein-A gold. High labeling is observed on a subpopulation of FLDF (c), and is completely abolished after depletion of membrane cholesterol with MβCD (d). (e) Low-density membranes were first isolated and then incubated with BCθ, followed by immunolabeling. A larger number of these membranes (≈30%) bound the toxin as compared with the isolation after BCθ binding. (Bars:a and b, 200 nm;c_–_e, 250 nm.)
Figure 4
Cholesterol depletion with 2OHpβCD completely reduces BCθ binding to FLDF. Platelets were incubated with 20 mM 2OHpβCD or buffer alone, followed by incubation with BCθ. Cell homogenates were fractionated into a sucrose gradient and analyzed as in Fig. 2. (a) BCθ binding to cholesterol-depleted (20 mM) and control (0 mM) platelets (Tx-treated). (b) Sphingomyelin distribution in cholesterol-depleted platelets treated with (+Tx) or without Tx (-Tx). (c) Cholesterol distribution in 2OHpβCD-treated (closed circles) or untreated (open circles) samples (+Tx). (d) The distribution of tyrosine-kinases in FLDF and bottom fractions (+Tx) of 2OHpβCD-treated (20mM CD) and untreated (0mM CD) platelets. T, total platelets. Distribution of sphingomyelin and membrane proteins was determined in platelets that were not incubated with BCθ as detailed in Fig. 2's legend.
Figure 5
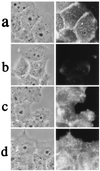
Labeling of A431 cells with BCθ or filipin. Monolayer A431 cells were incubated with serum-free DMEM for 2 h, fixed, and then incubated with either BCθ or filipin. BCθ-treated cells were then incubated with cy3-avidin. (a_–_d) Binding of BCθ (a and b) or filipin (c and d) to 2OHpβCD-treated cells (b and_d_) or untreated controls (a and_c_). Depletion of cholesterol completely abolished BCθ binding, whereas filipin binding was retained significantly. (Left) Phase contrast; (Right) fluorescence staining.
Similar articles
- Function of Platelet Glycosphingolipid Microdomains/Lipid Rafts.
Komatsuya K, Kaneko K, Kasahara K. Komatsuya K, et al. Int J Mol Sci. 2020 Aug 2;21(15):5539. doi: 10.3390/ijms21155539. Int J Mol Sci. 2020. PMID: 32748854 Free PMC article. Review. - Perfringolysin O, a cholesterol-binding cytolysin, as a probe for lipid rafts.
Ohno-Iwashita Y, Shimada Y, Waheed AA, Hayashi M, Inomata M, Nakamura M, Maruya M, Iwashita S. Ohno-Iwashita Y, et al. Anaerobe. 2004 Apr;10(2):125-34. doi: 10.1016/j.anaerobe.2003.09.003. Anaerobe. 2004. PMID: 16701509 - Biotinylated theta-toxin derivative as a probe to examine intracellular cholesterol-rich domains in normal and Niemann-Pick type C1 cells.
Sugii S, Reid PC, Ohgami N, Shimada Y, Maue RA, Ninomiya H, Ohno-Iwashita Y, Chang TY. Sugii S, et al. J Lipid Res. 2003 May;44(5):1033-41. doi: 10.1194/jlr.D200036-JLR200. Epub 2003 Feb 1. J Lipid Res. 2003. PMID: 12562855 - Gamma-secretase activity is present in rafts but is not cholesterol-dependent.
Wada S, Morishima-Kawashima M, Qi Y, Misono H, Shimada Y, Ohno-Iwashita Y, Ihara Y. Wada S, et al. Biochemistry. 2003 Dec 2;42(47):13977-86. doi: 10.1021/bi034904j. Biochemistry. 2003. PMID: 14636066 - Cholesterol reporter molecules.
Gimpl G, Gehrig-Burger K. Gimpl G, et al. Biosci Rep. 2007 Dec;27(6):335-58. doi: 10.1007/s10540-007-9060-1. Biosci Rep. 2007. PMID: 17668316 Review.
Cited by
- GsdmD p30 elicited by caspase-11 during pyroptosis forms pores in membranes.
Aglietti RA, Estevez A, Gupta A, Ramirez MG, Liu PS, Kayagaki N, Ciferri C, Dixit VM, Dueber EC. Aglietti RA, et al. Proc Natl Acad Sci U S A. 2016 Jul 12;113(28):7858-63. doi: 10.1073/pnas.1607769113. Epub 2016 Jun 23. Proc Natl Acad Sci U S A. 2016. PMID: 27339137 Free PMC article. - Function of Platelet Glycosphingolipid Microdomains/Lipid Rafts.
Komatsuya K, Kaneko K, Kasahara K. Komatsuya K, et al. Int J Mol Sci. 2020 Aug 2;21(15):5539. doi: 10.3390/ijms21155539. Int J Mol Sci. 2020. PMID: 32748854 Free PMC article. Review. - More Than a Pore: The Interplay of Pore-Forming Proteins and Lipid Membranes.
Ros U, García-Sáez AJ. Ros U, et al. J Membr Biol. 2015 Jun;248(3):545-61. doi: 10.1007/s00232-015-9820-y. Epub 2015 Jun 19. J Membr Biol. 2015. PMID: 26087906 Review. - Sterol carrier protein-2: new roles in regulating lipid rafts and signaling.
Schroeder F, Atshaves BP, McIntosh AL, Gallegos AM, Storey SM, Parr RD, Jefferson JR, Ball JM, Kier AB. Schroeder F, et al. Biochim Biophys Acta. 2007 Jun;1771(6):700-18. doi: 10.1016/j.bbalip.2007.04.005. Epub 2007 Apr 12. Biochim Biophys Acta. 2007. PMID: 17543577 Free PMC article. Review. - Evidence that membrane rafts are not required for the action of Clostridium perfringens enterotoxin.
Caserta JA, Hale ML, Popoff MR, Stiles BG, McClane BA. Caserta JA, et al. Infect Immun. 2008 Dec;76(12):5677-85. doi: 10.1128/IAI.00854-08. Epub 2008 Sep 22. Infect Immun. 2008. PMID: 18809663 Free PMC article.
References
- Hooper N M. Curr Biol. 1998;8:114–116. - PubMed
- Simons K, Ikonen E. Nature (London) 1997;387:569–572. - PubMed
- Brown D A, London E. Annu Rev Cell Dev Biol. 1998;14:111–136. - PubMed
- Brown D A, London E. J Biol Chem. 2000;275:17221–17224. - PubMed
- Fivaz M, Abrami L, van der Goot F G. Trends Cell Biol. 1999;9:212–213. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous