Lack of neurotrophin-4 causes selective structural and chemical deficits in sympathetic ganglia and their preganglionic innervation - PubMed (original) (raw)
Lack of neurotrophin-4 causes selective structural and chemical deficits in sympathetic ganglia and their preganglionic innervation
A Roosen et al. J Neurosci. 2001.
Abstract
Neurotrophin-4 (NT-4) is perhaps the still most enigmatic member of the neurotrophin family. We show here that NT-4 is expressed in neurons of paravertebral and prevertebral sympathetic ganglia, i.e., the superior cervical (SCG), stellate (SG), and celiac (CG) ganglion. Mice deficient for NT-4 showed a significant reduction (20-30%) of preganglionic sympathetic neurons in the intermediolateral column (IML) of the thoracic spinal cord. In contrast, neuron numbers in the SCG, SG, and CG were unchanged. Numbers of axons in the thoracic sympathetic trunk (TST) connecting the SG with lower paravertebral ganglia were also reduced, whereas axon numbers in the cervical sympathetic trunk (CST) were unaltered. Axon losses in the TST were paralleled by losses of synaptic terminals on SG neurons visualized by electron microscopy. Furthermore, immunoreactivity for the synaptic vesicle antigen SV2 was clearly reduced in the SG and CG. Levels of catecholamines and tyrosine hydroxylase immunoreactivity were dramatically reduced in the SG and the CG but not in the SCG. Despite this severe phenotype in the sympathetic system, blood pressure levels were not reduced and displayed a pattern more typical of deficits in baroreceptor afferents. Numbers of IML neurons were unaltered at postnatal day 4, suggesting a postnatal requirement for their maintenance. In light of these and previous data, we hypothesize that NT-4 provided by postganglionic sympathetic neurons is required for establishing and/or maintaining synapses of IML neurons on postganglionic cells. Impairment of synaptic connectivity may consequently reduce impulse flow, causing a reduction in transmitter synthesis in postganglionic neurons.
Figures
Fig. 1.
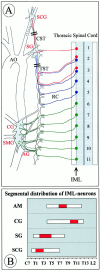
Anatomy of preganglionic and postganglionic structures in the sympathetic nervous system relevant to the present study (A). Cell bodies of preganglionic neurons are located in the IML at distinct levels of the spinal cord and project through rami communicantes (RC) to paravertebral and prevertebral sympathetic ganglia. We have analyzed IML neurons at levels T1–T5 that project to the paravertebral SCG and the SG, respectively. IML neurons projecting to the prevertebral CG and the superior mesenteric ganglion (SMG), which were also analyzed, cluster at spinal cord segments T10–T12. In our study, we did not make an attempt to separate the intimately associated CG and SMG; however, we did not include the aortorenal ganglion (AG). The adrenal gland and its preganglionic neurons, which peak in the IML T7–T10, are not shown. AO, Aorta.Double bars through the CST and TST mark portions that were analyzed for numbers of axons. B shows the distribution of IML neurons projecting to the adrenal medulla (AM) and various ganglia along the spinal cord axis according to Strack et al. (1988). The red portion of the bar indicates the peak accumulation of IML neurons for the respective targets.
Fig. 2.
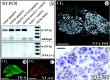
Expression of NT-4 mRNA and protein revealed by RT-PCR (A; 36 cycles), in situ hybridization (ISH) of the CG (D, E), and IR of the CG (C). B shows a consecutive section to C stained for TH. A, RT-PCR demonstrates expression of NT-4 mRNA in skeletal muscle (as a positive control) in the celiac, stellate, and superior cervical ganglion. Respective signals are absent in NT-4 mouse mutants. D,In situ hybridization shows that neuronal perikarya in the CG are prominent sites of expression of NT-4 mRNA.E, A higher magnifications reveals localization of grains over large neuronal cell bodies. B,C, TH- and NT-4-IR in consecutive sections of a CG reveal the presence of NT-4 protein in catecholaminergic sympathetic neurons. Scale bars: B–D, 500 μm; E, 50 μm.
Fig. 3.
Fluoro-Gold labeling (A, B) and quantitative evaluation (C–E) of IML neurons in wild-type and_NT-4_-deficient mice. A and _B_show longitudinal sections through the IML and adjacent LF at T1–T5, displaying Fluoro-Gold-labeled preganglionic sympathetic neurons.C–E, Adult mice lacking NT-4_show significant losses of Fluoro-Gold-labeled IML neurons, the extent of which varies in different spinal cord segments. Data are given as mean ± SEM. p values derived from Student's_t test are as indicated. Scale bars, 50 μm.
Fig. 4.
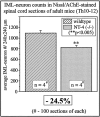
IML neuron counts in Nissl- and AChE-stained spinal cord sections (segments T10–T12) of wild-type and_NT-4_-deficient mice.
Fig. 5.
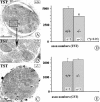
Qualitative and quantitative evaluation of axons in the TST and CST (compare with Fig. 1). A–C show electron micrographs of cross-sections through the TST. The majority of axons represent preganglionic connections; preganglionic axons in rodents are mostly unmyelinated. D and _E_show the results of axon counts in the TST (D) and CST (E) of adult wild-type and_NT-4_-deficient mice. Whereas numbers of axons in the CST do not significantly differ in wild-type and mutant mice, axon numbers of the TST are reduced by 22% in mutant mice compared with wild-type littermates. Data are given as mean ± SEM. Scale bars:A, C, 50 μm; B, 10 μm.
Fig. 6.
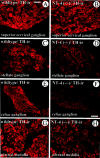
SV2, a synaptic vesicle antigen in preganglionic terminals, is distinctly affected in the SCG (A,B), SG (C, D), and CG (E, F) of NT-4 mutant mice. SV2-IR is localized in structures, presumed axon terminals on dendrites, surrounding the large cell bodies of sympathetic ganglion cells, which exhibit only background fluorescence. SV2-IR is clearly reduced in axon terminals of the SG and CG, but not the SCG, of_NT-4_-deficient mice (B, D,F) compared with wild-type littermates (A, C, E). Scale bar, 100 μm.
Fig. 7.
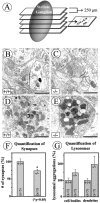
Numbers of synapses (B,C, F) and dense bodies (D, E, G) were evaluated in electron micrographs taken from five sections through the SG, with a distance of 250 μm, within an area of 0.74 × 0.74 mm per section (A) from one _NT-4_-deficient mouse and one wild-type littermate. Criteria for synapses were presynaptic and postsynaptic membrane specializations and accumulations of synaptic vesicles in the active zone (B,C). Although ultrastructural features of synaptic nerve endings were not overtly changed in NT-4 knock-outs compared with wild-type animals (B, C), there were significant quantitative differences in numbers of synapses (F). Numbers of lysosomes in neuronal cell bodies and dendrites seemed to be augmented in the NT-4 knock-outs, however, without becoming significant (G). Scale bars: B, C, 1 μm; D,E, 2 μm.
Fig. 8.
Comparison of TH-IR in the SCG (A,B), SG (C, D), CG (E, F), and adrenal medulla (G, H) of_NT-4_-deficient mice and wild-type littermates. Although TH-IR is not overtly altered in the SCG, the SG, CG, and adrenal medulla reveal a qualitative reduction of TH staining intensity. Scale bars: A–D, G, H, 50 μm;E, F, 100 μm.
Fig. 9.
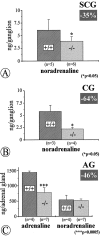
Quantitative determinations of catecholamines by HPLC–electrochemical detection in the SCG (A), CG (B), and adrenal gland (AG; C) of_NT-4_-deficient mice and wild-type littermates reveal significant losses of noradrenaline in the SCG and, more severely, in the CG, and losses of adrenaline in the adrenal gland. Data are given as means ± SEM.
Fig. 10.
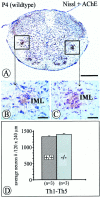
Quantification of acetylcholinesterase-positive, Nissl-stained IML neurons within an area of 120 × 240 μm of the intermediate gray in the spinal cord at P4. Numbers of IML neurons in_NT-4_-deficient mice (C, D) were not significantly reduced compared with controls (B, D) at this age. Data are given as mean ± SEM. Scale bars: A, 500 μm;B, C, 50 μm.
Fig. 11.
RT-PCR for BDNF (32 cycles) and_actin_ (24 cycles) mRNAs. Celiac, stellate, and superior cervical ganglia were separately pooled from four_NT-4_-deficient mice and four wild-type littermates. The same cerebellar sample was run on both gels, making signals on both gels directly comparable. BDNF mRNA signals were standardized using the β-actin signal. Signal intensity was quantified using computer software (NIH Image). Analysis of signal intensities suggests upregulation of BDNF mRNA in the CG and SG (both affected by the NT-4 knock-out) but not in the SCG (not affected).
Similar articles
- TrkB and neurotrophin-4 are important for development and maintenance of sympathetic preganglionic neurons innervating the adrenal medulla.
Schober A, Wolf N, Huber K, Hertel R, Krieglstein K, Minichiello L, Kahane N, Widenfalk J, Kalcheim C, Olson L, Klein R, Lewin GR, Unsicker K. Schober A, et al. J Neurosci. 1998 Sep 15;18(18):7272-84. doi: 10.1523/JNEUROSCI.18-18-07272.1998. J Neurosci. 1998. PMID: 9736648 Free PMC article. - Gamma-aminobutyric acid-containing sympathetic preganglionic neurons in rat thoracic spinal cord send their axons to the superior cervical ganglion.
Ito T, Hioki H, Nakamura K, Tanaka Y, Nakade H, Kaneko T, Iino S, Nojyo Y. Ito T, et al. J Comp Neurol. 2007 May 1;502(1):113-25. doi: 10.1002/cne.21309. J Comp Neurol. 2007. PMID: 17335042 - Morphology of synapses in the autonomic nervous system.
Smolen AJ. Smolen AJ. J Electron Microsc Tech. 1988 Oct;10(2):187-204. doi: 10.1002/jemt.1060100205. J Electron Microsc Tech. 1988. PMID: 3068334 Review.
Cited by
- Retrograde influences of SCG axotomy on uninjured preganglionic neurons.
Gannon SM, Hawk K, Walsh BF, Coulibaly A, Isaacson LG. Gannon SM, et al. Brain Res. 2018 Jul 15;1691:44-54. doi: 10.1016/j.brainres.2018.04.014. Epub 2018 Apr 19. Brain Res. 2018. PMID: 29679543 Free PMC article. - Neurotrophin-regulated signalling pathways.
Reichardt LF. Reichardt LF. Philos Trans R Soc Lond B Biol Sci. 2006 Sep 29;361(1473):1545-64. doi: 10.1098/rstb.2006.1894. Philos Trans R Soc Lond B Biol Sci. 2006. PMID: 16939974 Free PMC article. Review. - Development of the vagal innervation of the gut: steering the wandering nerve.
Ratcliffe EM, Farrar NR, Fox EA. Ratcliffe EM, et al. Neurogastroenterol Motil. 2011 Oct;23(10):898-911. doi: 10.1111/j.1365-2982.2011.01764.x. Epub 2011 Aug 18. Neurogastroenterol Motil. 2011. PMID: 21851506 Free PMC article. Review. - Cell loss and autophagy in the extra-adrenal chromaffin organ of Zuckerkandl are regulated by glucocorticoid signalling.
Schober A, Parlato R, Huber K, Kinscherf R, Hartleben B, Huber TB, Schütz G, Unsicker K. Schober A, et al. J Neuroendocrinol. 2013 Jan;25(1):34-47. doi: 10.1111/j.1365-2826.2012.02367.x. J Neuroendocrinol. 2013. PMID: 23078542 Free PMC article. - Heterogeneous requirement of NGF for sympathetic target innervation in vivo.
Glebova NO, Ginty DD. Glebova NO, et al. J Neurosci. 2004 Jan 21;24(3):743-51. doi: 10.1523/JNEUROSCI.4523-03.2004. J Neurosci. 2004. PMID: 14736860 Free PMC article.
References
- Anderson CR, Edwards SL. Intraperitoneal injections of Fluoro-Gold reliably labels all sympathetic preganglionic neurons in the rat. J Neurosci Methods. 1994;53:137–141. - PubMed
- Andrä J, Lojda Z. A histochemical method for the demonstration of acetylcholinesterase activity using semipermeable membranes. Histochemistry. 1986;84:575–579. - PubMed
- Barbacid M. The Trk family of neurotrophin receptors. J Neurobiol. 1994;25:1386–1403. - PubMed
- Barde YA. Trophic factors and neuronal survival. Neuron. 1989;2:1525–1534. - PubMed
- Blottner D, Unsicker K. Maintenance of intermediolateral spinal cord neurons by fibroblast growth factor administered to the medullectomized rat adrenal gland: dependence on intact organ innervation and cellular organization of implants. Eur J Neurosci. 1990;2:378–382. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials