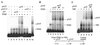p53 Stimulates TFIID-TFIIA-promoter complex assembly, and p53-T antigen complex inhibits TATA binding protein-TATA interaction - PubMed (original) (raw)
p53 Stimulates TFIID-TFIIA-promoter complex assembly, and p53-T antigen complex inhibits TATA binding protein-TATA interaction
J Xing et al. Mol Cell Biol. 2001 Jun.
Abstract
Simian virus 40 large T antigen has been shown to inhibit p53-mediated transcription once tethered to p53-responsive promoters through interaction with p53. In this study we report that p53 stimulates transcription by enhancing the recruitment of the basal transcription factors, TFIIA and TFIID, on the promoter (the DA complex) and by inducing a conformational change in the DA complex. Significantly, we have demonstrated that T antigen inhibits p53-mediated transcription by blocking this ability of p53. We investigated the mechanism for this inhibition and found that DA complex formation was resistant to T-antigen repression when the TFIID-DNA complex was formed prior to addition of p53-T antigen complex, indicating that the T antigen, once tethered to the promoter by p53, targets TFIID. Further, we have shown that the p53-T antigen complex prevents the TATA binding protein from binding to the TATA box. Thus, these data suggest a detailed mechanism by which p53 activates transcription and by which T antigen inhibits p53-mediated transcription.
Figures
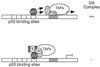
FIG. 1
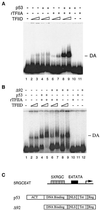
p53 stimulates TFIID-TFIIA-promoter complex assembly. (A) Mg-EMSA of p53-induced DA complex formation. Purified TFIID complex, recombinant TFIIA (rTFIIA), and p53 (as indicated above each lane) were incubated with the 5RGCE4T probe for 30 min at 30°C. The amount of each factor added to the Mg-EMSA reactions corresponds to 0.5 or 1 μl of TFIID, 20 ng of rTFIIA, and 100 ng of p53. The specific position of the DA complex is indicated on the right. (B) ΔN92 (Δ92) fails to induce DA complex formation. The amount of each factor added to the Mg-EMSA reactions corresponds to 0.5 or 1 μl of TFIID, 20 ng of rTFIIA, 100 ng of p53, or 100 ng of ΔN92. The position of the DA complex is indicated on the right. (C) Schematic diagram of the 5RGCE4T template (transcription initiation site designated by the right-angle arrow), p53, and ΔN92 used Act, activation domain; NLS, nuclear localization signal; Tet, tetramerization; Reg, regulatory domain.
FIG. 2
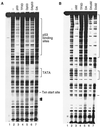
The conformational change of the DA complex is necessary for p53 transactivation. (A) p53, TFIIA, and TFIID cooperate to form a stable TFIID footprint on the promoter. In vitro DNase I footprint reactions were carried out as described in Materials and Methods. The amount of each factor added to the DNase I footprint reactions (as indicated above each lane) corresponds to 5 μl of TFIID, 100 ng of recombinant TFIIA, and 500 ng of p53. The positions of the p53 binding sites, the TATA box, and the transcription (Txn) start site are indicated on the right. Arrows denote hypersensitive sites. (B) ΔN92 (Δ92) fails to induce the conformational change of the DA complex on the promoter. The amount of each factor added to the DNase I footprint reactions (as indicated above each lane) corresponds to 5 μl of TFIID, 100 ng of recombinant TFIIA, 500 ng of p53, or 500 ng of ΔN92. The positions of the p53 binding sites, the TATA box, and the transcription start site are indicated on the right.
FIG. 3
p53-T complex (p53/T) is incapable of stimulating DA complex assembly. (A) p53-T complex fails to induce DA complex formation. Mg-EMSA reactions were carried out as described in Materials and Methods. The amount of each factor added to the Mg-EMSA reactions corresponds to 1 μl of TFIID, 20 ng of recombinant TFIIA (rTFIIA), 50 or 100 ng of p53, and 3 or 6 μl of p53-T complex (containing 100 or 200 ng of p53). The positions of the DA complex, p53-T–DNA, and p53-DNA are indicated on the right. (B) Experiments were performed as for panel A but in the presence of 100 ng of anti-TBP antibody (α-TBP, N-12). (C) p53 purified from mouse cells is capable of stimulating DA complex assembly. Mg-EMSA reactions were carried out as described. The amount of each factor added to the Mg-EMSA reactions (as indicated above each lane) corresponds to 1 μl of TFIID, 20 ng of rTFIIA, 100 ng of p53, and 100 ng of p53-T without T antigen (p53/T −T). The position of the DA complex is indicated on the left. (D) A silver-stained SDS-PAGE gel is shown. Lane 1, 200 ng of HA-tagged human p53; lane 2, 100 ng of HA-tagged ΔN92; lane 3, 1 μl of purified p53-T antigen complex corresponding to 30 ng of p53; lane 4, 1 μl of purified p53-T complex without T antigen corresponding to 30 ng of p53. The sizes (in kilodaltons) of molecular mass standards are indicated on the left.
FIG. 4
Interaction with TFIIA is not sufficient for the inhibition of DA complex assembly. (A) p53 and ΔN92 (Δ92) were translated in vitro with [35S]methionine, and p53-T complex (p53/T) was radiolabeled in vivo in the presence of [35S]methionine and purified with Pab 421 antibody as described in Materials and Methods. The labeled proteins were either incubated with GST or GST-TFIIA immobilized on glutathione Sepharose beads (left panel) or with TFIID which was immobilized on protein A-Sepharose beads using TAFII250 antibody (6B3, right panel, TFIID IP). After being washed, bound proteins were analyzed by SDS-PAGE followed by autoradiography of the proteins retained on the beads. (B) Experiments were performed as for panel A but using GST-TBP. The relative amount of bound protein was quantitated using a PhosphorImager and ImageQuant software and is presented as a percentage of the total radiolabeled protein.
FIG. 5
T antigen targets TFIID to inhibit DA complex assembly. (A) p53-T complex (p53/T) prevents TFIID from binding to the promoter. Mg-EMSA reactions were carried out with TFIID complex, p53, and p53-T complex (as indicated above each lane) using the 5RGCE4T probe. (B) A preassembled complex containing DNA and TFIID is partially resistant to p53-T complex inhibition. TFIID was incubated with the probe for 30 min at 30°C in either the presence or absence of increasing amounts of p53-T complex. p53-T complex was then added to the reaction mixtures in lanes 5 and 6 and was incubated for an additional 30 min prior to the Mg-EMSA. (C) A preassembled complex containing DNA and TBP is not resistant to p53-T complex inhibition. Experiments were performed as for panel A but with 10 ng of TBP instead of TFIID. All preincubation Mg-EMSA reactions were carried out for 60 min.
FIG. 6
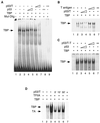
T antigen prevents TBP from binding to the TATA box. (A) EMSA reactions were carried out with 10 ng of TBP, 10 or 20 ng of p53, and 0.3 or 0.6 μl of p53-T complex corresponding to 10 or 20 ng of p53 for 30 min at 30°C. The reaction mixtures were analyzed on a 3% polyacrylamide gel. The position of the TBP-DNA complex is indicated on the left. Mut Olig, E1B TATA-oligo probe. (B) T antigen alone has no effect on TBP binding. Experiments were performed as for panel A but with 10 or 20 ng of T antigen. (C) Mouse p53 alone has no effect on TBP binding. Experiments were performed as for panel A but with 10 or 20 ng of mouse p53 (p53/T−T). (D) A preassembled complex containing DNA, TFIIA, and TBP is partially resistant to p53-T complex inhibition. TBP, TFIIA, and the E1B probe were preincubated for 5, 15, or 30 min before the addition of p53-T complex. The time at which p53-T complex (p53/T) was added is indicated above. All preincubation EMSA reactions were carried out for 60 min. The arrows on the left indicate the positions of the TBP-DNA and TBP-IIA-DNA complexes.
FIG. 7
A model for how p53-T complex inhibits p53-mediated transcription. (Top) p53 stimulates the TFIID-TFIIA-promoter complex formation. (Bottom) T antigen represses transcription when tethered to the promoter by p53. This could occur through a conformational change of TBP within the TFIID-TFIIA-promoter complex or could be due to the exclusion of the ability of TFIIA to function as an antirepressor.
Similar articles
- Simian virus 40 large T antigen stabilizes the TATA-binding protein-TFIIA complex on the TATA element.
Damania B, Lieberman P, Alwine JC. Damania B, et al. Mol Cell Biol. 1998 Jul;18(7):3926-35. doi: 10.1128/MCB.18.7.3926. Mol Cell Biol. 1998. PMID: 9632777 Free PMC article. - Transcription factor IIA derepresses TATA-binding protein (TBP)-associated factor inhibition of TBP-DNA binding.
Ozer J, Mitsouras K, Zerby D, Carey M, Lieberman PM. Ozer J, et al. J Biol Chem. 1998 Jun 5;273(23):14293-300. doi: 10.1074/jbc.273.23.14293. J Biol Chem. 1998. PMID: 9603936 - Requirement for transcription factor IIA (TFIIA)-TFIID recruitment by an activator depends on promoter structure and template competition.
Lieberman PM, Ozer J, Gürsel DB. Lieberman PM, et al. Mol Cell Biol. 1997 Nov;17(11):6624-32. doi: 10.1128/MCB.17.11.6624. Mol Cell Biol. 1997. PMID: 9343426 Free PMC article. - Mechanisms of transcriptional activation and repression can both involve TFIID.
Manley JL, Um M, Li C, Ashali H. Manley JL, et al. Philos Trans R Soc Lond B Biol Sci. 1996 Apr 29;351(1339):517-26. doi: 10.1098/rstb.1996.0050. Philos Trans R Soc Lond B Biol Sci. 1996. PMID: 8735274 Review. - TBP-associated factors (TAFIIs): multiple, selective transcriptional mediators in common complexes.
Green MR. Green MR. Trends Biochem Sci. 2000 Feb;25(2):59-63. doi: 10.1016/s0968-0004(99)01527-3. Trends Biochem Sci. 2000. PMID: 10664584 Review.
Cited by
- The p53/Adipose-Tissue/Cancer Nexus.
Zwezdaryk K, Sullivan D, Saifudeen Z. Zwezdaryk K, et al. Front Endocrinol (Lausanne). 2018 Aug 14;9:457. doi: 10.3389/fendo.2018.00457. eCollection 2018. Front Endocrinol (Lausanne). 2018. PMID: 30158901 Free PMC article. Review. - Activity of the upstream TATA-less promoter of the p21(Waf1/Cip1) gene depends on transcription factor IIA (TFIIA) in addition to TFIIA-reactive TBP-like protein.
Suzuki H, Maeda R, Nakadai T, Tamura TA. Suzuki H, et al. FEBS J. 2014 Jul;281(14):3126-37. doi: 10.1111/febs.12848. Epub 2014 Jun 6. FEBS J. 2014. PMID: 24835508 Free PMC article. - Transcriptional regulation by p53.
Beckerman R, Prives C. Beckerman R, et al. Cold Spring Harb Perspect Biol. 2010 Aug;2(8):a000935. doi: 10.1101/cshperspect.a000935. Epub 2010 Apr 28. Cold Spring Harb Perspect Biol. 2010. PMID: 20679336 Free PMC article. Review. - Redox state of tumor suppressor p53 regulates its sequence-specific DNA binding in DNA-damaged cells by cysteine 277.
Buzek J, Latonen L, Kurki S, Peltonen K, Laiho M. Buzek J, et al. Nucleic Acids Res. 2002 Jun 1;30(11):2340-8. doi: 10.1093/nar/30.11.2340. Nucleic Acids Res. 2002. PMID: 12034820 Free PMC article. - Versatile functions of p53 protein in multicellular organisms.
Chumakov PM. Chumakov PM. Biochemistry (Mosc). 2007 Dec;72(13):1399-421. doi: 10.1134/s0006297907130019. Biochemistry (Mosc). 2007. PMID: 18282133 Free PMC article. Review.
References
- Bargonetti J, Reynisdottir I, Friedman P, Prives C. Site-specific binding of wild-type p53 to cellular DNA is inhibited by SV40 T antigen and mutant p53. Genes Dev. 1992;6:1886–1898. - PubMed
- Carbone M, Rizzo P, Pass H I. Simian virus 40, poliovaccines and human tumors: a review of recent developments. Oncogene. 1997;15:1877–1888. - PubMed
- Carbone M, Rizzo P, Grimley P M, Procopio A, Mew D J Y, Shridhar V, de Bartolomeis A, Esposito V, Giuliano M T, Steinberg S M, Levine A, Giordano A, Pass H I. Simian virus-40 large T-antigen binds p53 in human mesotheliomas. Nat Med. 1997;3:908–912. - PubMed
- Chi T, Lieberman P, Ellwood K, Carey M. A general mechanism for transcription synergy by eukaryotic activators. Nature. 1995;377:254–257. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous