Feedback inhibition of the unfolded protein response by GADD34-mediated dephosphorylation of eIF2alpha - PubMed (original) (raw)
Feedback inhibition of the unfolded protein response by GADD34-mediated dephosphorylation of eIF2alpha
I Novoa et al. J Cell Biol. 2001.
Abstract
Phosphorylation of the alpha subunit of eukaryotic translation initiation factor 2 (eIF2alpha) on serine 51 integrates general translation repression with activation of stress-inducible genes such as ATF4, CHOP, and BiP in the unfolded protein response. We sought to identify new genes active in this phospho-eIF2alpha-dependent signaling pathway by screening a library of recombinant retroviruses for clones that inhibit the expression of a CHOP::GFP reporter. A retrovirus encoding the COOH terminus of growth arrest and DNA damage gene (GADD)34, also known as MYD116 (Fornace, A.J., D.W. Neibert, M.C. Hollander, J.D. Luethy, M. Papathanasiou, J. Fragoli, and N.J. Holbrook. 1989. Mol. Cell. Biol. 9:4196-4203; Lord K.A., B. Hoffman-Lieberman, and D.A. Lieberman. 1990. Nucleic Acid Res. 18:2823), was isolated and found to attenuate CHOP (also known as GADD153) activation by both protein malfolding in the endoplasmic reticulum, and amino acid deprivation. Despite normal activity of the cognate stress-inducible eIF2alpha kinases PERK (also known as PEK) and GCN2, phospho-eIF2alpha levels were markedly diminished in GADD34-overexpressing cells. GADD34 formed a complex with the catalytic subunit of protein phosphatase 1 (PP1c) that specifically promoted the dephosphorylation of eIF2alpha in vitro. Mutations that interfered with the interaction with PP1c prevented the dephosphorylation of eIF2alpha and blocked attenuation of CHOP by GADD34. Expression of GADD34 is stress dependent, and was absent in PERK(-)/- and GCN2(-)/- cells. These findings implicate GADD34-mediated dephosphorylation of eIF2alpha in a negative feedback loop that inhibits stress-induced gene expression, and that might promote recovery from translational inhibition in the unfolded protein response.
Figures
Figure 1
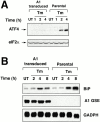
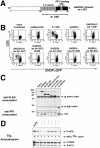
A1 is a GSE attenuating CHOP expression by ER stress and amino acid deprivation. (A) GFP expression levels analyzed by FACS® in parental _CHOP::GFP_-containing CHO cells and in cells transduced by the A1 retroviral clone. Cells were untreated and cultured in complete media (UT), cultured for 16 h in media lacking leucine (−Leu), or exposed to tunicamycin (2 μg/ml) for 16 h (Tm). The fold increase in GFP compared with the untreated cells is indicated. (B) Immunoblot of endogenous CHOP in the parental and A1-transduced cells after exposure to tunicamycin (2 μg/ml) or leucine-free media (upper panel). Anti-eIF2α immunoblot of the same membrane serves as a control of protein loading (lower panel).
Figure 2
The A1 GSE interferes with a step common to the activation of BiP and ATF4 in the UPR. (A) Immunoblot of ATF4 in tunicamycin-treated parental and A1-transduced CHO cells (upper panel). Anti-eIF2α immunoblot of the same membrane serves as a control of protein loading (lower panel). (B) Northern blot of RNA from tunicamycin-treated parental and A1-transduced CHO cells. The blot was hybridized sequentially with BiP, the insert of the A1 retrovirus, and GAPDH radiolabeled probes.
Figure 3
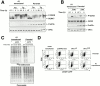
The A1 GSE interferes with eIF2α phosphorylation and downstream signaling but does not block stress-activation of the eIF2α kinases PERK and GCN2. (A) Immunoblot of PERK immunoprecipitated from parental and A1-transduced cells after treatment with tunicamycin (2 μg/ml, Tm) or thapsigargin (100 nM, Tg) (upper panel). The positions of the higher mobility, inactive form of the protein (PERK0) and the lower mobility, phosphorylated active form of the protein (P-PERK) are indicated. Immunoblot of the same lysate with antisera that detects eIF2α phosphorylated on serine 51 (P-eIF2α), or total eIF2α. (B) Immunoblot of GCN2 immunoprecipitated from parental and A1-transduced cells cultured in leucine-deficient media where indicated (−Leu). GCN2 is revealed by immunoblot with an antiserum that detects the phosphorylated, active form of the kinase (P-GCN2), or an antiserum that reacts with all forms of the kinase (GCN2) (upper panels). Immunoblot of the same lysates with antisera to eIF2α as in A (lower panels). (C) Autoradiography of a SDS-PAGE gel of whole cell lysates from parental and A1-transduced CHO cells that had been treated with DTT (2 mM) for the indicated period of time to rapidly induce ER stress, and that had been pulsed with [35S]methionine. The lower panel shows a Coomassie blue stain of the same gel to control for equal loading of the lanes. (D) FACS® analysis of GFP activity in A1-transduced or parental CHOP::GFP CHO cells mock-transfected or transfected with expression plasmids that encode the CD2 cell surface marker alone, CD2 and wild-type eIF2α (eIF2αWT), or CD2 and S51D mutant eIF2α (eIF2αS51D). Note the population of double positive (CD2+ and GFP+) cells present only in the pool transfected with the mutant eIF2α (rectangle).
Figure 4
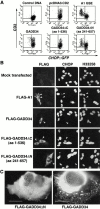
GADD34 inhibits CHOP activation in the UPR. (A) FACS® analysis of CHO cells expressing the CHOP::GFP reporter and transfected with CD2-expressing plasmids that coexpress the indicated derivatives of mouse GADD34, the A1 GSE, or no additional protein. The cells were treated with tunicamycin (2 μg/ml, 16 h) to activate CHOP::GFP. Note the presence of CD2-positive and GFP-negative cells (rectangle) in the pools transfected with A1, wild-type GADD34, and GADD34ΔN. (B) Immunostaining of mouse embryonic fibroblast cells transfected with expression vectors encoding FLAG-tagged A1 or GADD34, and the indicated deletion mutants of GADD34. The cells were treated with tunicamycin (2 μg/ml, 8 h) and immunostained with an antibody to FLAG that detects the recombinant proteins and an antiserum to CHOP that detects endogenous CHOP. The karyophilic dye H33258 stains the nuclei of all the cells in the field. The arrows mark the cells expressing the recombinant GADD34 proteins. (C) High magnification (60×) view of transfected cells expressing the full-length mouse GADD34 or the ΔN deletion. The recombinant proteins are revealed by immunostaining of the FLAG epitope tag.
Figure 5
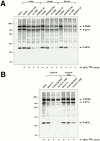
Cells expressing GADD34 have increased phosphatase activity directed towards eIF2α. (A) Autoradiogram revealing the in vitro dephosphorylation of radiolabeled eIF2α by lysates of cells expressing the A1 GSE or the mouse GADD34 COOH-terminal fragment (GADD34 ΔN). eIF2α in rabbit reticulocyte lysate was radiolabeled in vitro on serine 51 with GST-PERK, and incubated for the specified time with lysates of CHO cells expressing the indicated GADD34 derivatives. The position on the SDS-PAGE of radiolabeled eIF2α (P-eIF2α), GST-PERK (P-PERK), and an unidentified phosphoprotein of 110 kD present in the reticulocyte lysate (P-p110) are indicated. The latter two serve as controls for the specificity of the activity that dephosphorylates eIF2α. The radioactive signal associated with eIF2α is quantified in arbitrary units (eIF2α 32P-count). (B) Autoradiogram revealing the in vitro dephosphorylation of radiolabeled eIF2α by complexes immunoprecipitated from 293T cells transfected with epitope-tagged, full-length mouse GADD34 (FLAG-GADD34) or the A1 GSE (FLAG-A1 GSE), or mock-transfected cells (Mock Tx). The in vitro dephosphorylation assays were carried out in the absence or presence of phosphatase inhibitor (2 μM okadaic acid, +OA).
Figure 6
GADD34 inhibition of CHOP expression requires interaction with PP1. (A) Structure of mouse GADD34. The conserved repeat motifs are hatched, the COOH-terminal region with sequence similarity to HSV γ134.5 is colored black, and the peptide motif presumed to interact with PP1c is colored gray. (B) FACS® analysis of CHO cells expressing the CHOP::GFP reporter and transfected with CD2-expressing plasmids that coexpress the indicated derivatives of mouse GADD34, the A1 GSE, or no additional protein. The cells were treated with tunicamycin (2 μg/ml, 16 h) to activate CHOP::GFP. Note the presence of CD2-positive, GFP-negative cells (rectangle) in the pools transfected with A1, GADD34, GADD34ΔN, and GADD34 (aa 485–657), but not in the COOH-terminal deletions of GADD34 (aa 1–536, 241–562, 241–536) or in the V549E mutant form. (C) Anti-FLAG immunoprecipitation followed by immunoblot of proteins from lysates of cells transfected with FLAG-tagged GADD34 and the indicated derivatives as in B. The recombinant proteins in the immunoprecipitates were detected by immunoblotting with an antibody to the FLAG epitope (upper panel), and coimmunoprecipitating endogenous PP1c was detected by a specific polyclonal serum (bottom panel). The position of the precipitating immunoglobulin heavy and light chains and the PP1c band are indicated by arrows. (D) Autoradiogram of eIF2α that had been phosphorylated in vitro with 32P on serine 51 (P-eIF2α) after incubation for 15 min with lysates of cells transfected as in C (upper panel). The lane marked “-lysate” reports on the 32P–eIF2α signal from sample incubated under the same conditions in the absence of lysate. The radioactivity signal of the 32PeIF2α is quantified in arbitrary units below (eIF2α 32P-count). Autoradiogram of the signal from 32PGST-PERK and an unidentified radiolabeled phosphoprotein of 110 kD that were present in the preparation of 32PeIF2α and serve as control substrates for the phosphatase activity (lower panel).
Figure 7
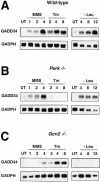
GADD34 expression depends on stress-induced eIF2α kinases. Northern blot analysis of GADD34 and GAPDH mRNA in (A) wild-type cells, (B) PERK−/− cells, and (C) GCN2−/− cells. Cells were exposed to tunicamycin (Tm, 2 μg/ml), the alkylating agent MMS (100 μg/ml), or cultured in the absence of leucine (−Leu) for the indicated period of time.
Figure 8
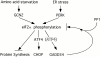
Model depicting GADD34's role in inhibiting signaling through the integrated stress response pathway. GADD34 is induced transcriptionally by the eIF2α kinases PERK and GCN2. It associates with PP1c to reduce levels of eIF2α phosphorylation and feed-back negatively on the pathway. ATF4 is a transcription factor that is activated translationally by eIF2α phosphorylation and plays a role in the transcriptional activation of CHOP in the integrated stress response (Harding et al. 2000a). We do not know if it plays a similar role in GADD34 activation and for this reason it is displayed by a shadowed font.
Similar articles
- Regulated translation initiation controls stress-induced gene expression in mammalian cells.
Harding HP, Novoa I, Zhang Y, Zeng H, Wek R, Schapira M, Ron D. Harding HP, et al. Mol Cell. 2000 Nov;6(5):1099-108. doi: 10.1016/s1097-2765(00)00108-8. Mol Cell. 2000. PMID: 11106749 - Stress-induced gene expression requires programmed recovery from translational repression.
Novoa I, Zhang Y, Zeng H, Jungreis R, Harding HP, Ron D. Novoa I, et al. EMBO J. 2003 Mar 3;22(5):1180-7. doi: 10.1093/emboj/cdg112. EMBO J. 2003. PMID: 12606582 Free PMC article. - Pausing to decide.
Niwa M, Walter P. Niwa M, et al. Proc Natl Acad Sci U S A. 2000 Nov 7;97(23):12396-7. doi: 10.1073/pnas.250476097. Proc Natl Acad Sci U S A. 2000. PMID: 11058174 Free PMC article. Review. No abstract available. - Coping with stress: eIF2 kinases and translational control.
Wek RC, Jiang HY, Anthony TG. Wek RC, et al. Biochem Soc Trans. 2006 Feb;34(Pt 1):7-11. doi: 10.1042/BST20060007. Biochem Soc Trans. 2006. PMID: 16246168 Review.
Cited by
- IRE1α-Dependent Decay of CReP/Ppp1r15b mRNA Increases Eukaryotic Initiation Factor 2α Phosphorylation and Suppresses Protein Synthesis.
So JS, Cho S, Min SH, Kimball SR, Lee AH. So JS, et al. Mol Cell Biol. 2015 Aug;35(16):2761-70. doi: 10.1128/MCB.00215-15. Epub 2015 Jun 1. Mol Cell Biol. 2015. PMID: 26031337 Free PMC article. - ER Stress-Mediated Signaling: Action Potential and Ca(2+) as Key Players.
Bahar E, Kim H, Yoon H. Bahar E, et al. Int J Mol Sci. 2016 Sep 15;17(9):1558. doi: 10.3390/ijms17091558. Int J Mol Sci. 2016. PMID: 27649160 Free PMC article. Review. - Murine cytomegalovirus targets transcription factor ATF4 to exploit the unfolded-protein response.
Qian Z, Xuan B, Chapa TJ, Gualberto N, Yu D. Qian Z, et al. J Virol. 2012 Jun;86(12):6712-23. doi: 10.1128/JVI.00200-12. Epub 2012 Apr 11. J Virol. 2012. PMID: 22496230 Free PMC article. - Signaling plasticity in the integrated stress response.
Boone M, Zappa F. Boone M, et al. Front Cell Dev Biol. 2023 Dec 7;11:1271141. doi: 10.3389/fcell.2023.1271141. eCollection 2023. Front Cell Dev Biol. 2023. PMID: 38143923 Free PMC article. Review. - PERK/eIF2α signaling protects therapy resistant hypoxic cells through induction of glutathione synthesis and protection against ROS.
Rouschop KM, Dubois LJ, Keulers TG, van den Beucken T, Lambin P, Bussink J, van der Kogel AJ, Koritzinsky M, Wouters BG. Rouschop KM, et al. Proc Natl Acad Sci U S A. 2013 Mar 19;110(12):4622-7. doi: 10.1073/pnas.1210633110. Epub 2013 Mar 7. Proc Natl Acad Sci U S A. 2013. PMID: 23471998 Free PMC article.
References
- Berlanga J.J., Santoyo J., De Haro C. Characterization of a mammalian homologue of the GCN2 eukaryotic initiation factor 2alpha kinase. Eur. J. Biochem. 1999;265:754–762. - PubMed
- Bertolotti A., Zhang Y., Hendershot L., Harding H., Ron D. Dynamic interaction of BiP and the ER stress transducers in the unfolded protein response. Nat. Cell Biol. 2000;2:326–332. - PubMed
- Brostrom C.O., Brostrom M.A. Regulation of translational initiation during cellular responses to stress. Prog. Nucleic Acid Res. Mol. Biol. 1998;58:79–125. - PubMed
- Camps M., Nichols A., Arkinstall S. Dual specificity phosphatasesa gene family for control of MAP kinase function. FASEB J. 2000;14:6–16. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 DK047119/DK/NIDDK NIH HHS/United States
- R01 ES008681/ES/NIEHS NIH HHS/United States
- ES08681/ES/NIEHS NIH HHS/United States
- R37 DK047119/DK/NIDDK NIH HHS/United States
- DK47119/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials