Focal contacts as mechanosensors: externally applied local mechanical force induces growth of focal contacts by an mDia1-dependent and ROCK-independent mechanism - PubMed (original) (raw)
Focal contacts as mechanosensors: externally applied local mechanical force induces growth of focal contacts by an mDia1-dependent and ROCK-independent mechanism
D Riveline et al. J Cell Biol. 2001.
Abstract
The transition of cell-matrix adhesions from the initial punctate focal complexes into the mature elongated form, known as focal contacts, requires GTPase Rho activity. In particular, activation of myosin II-driven contractility by a Rho target known as Rho-associated kinase (ROCK) was shown to be essential for focal contact formation. To dissect the mechanism of Rho-dependent induction of focal contacts and to elucidate the role of cell contractility, we applied mechanical force to vinculin-containing dot-like adhesions at the cell edge using a micropipette. Local centripetal pulling led to local assembly and elongation of these structures and to their development into streak-like focal contacts, as revealed by the dynamics of green fluorescent protein-tagged vinculin or paxillin and interference reflection microscopy. Inhibition of Rho activity by C3 transferase suppressed this force-induced focal contact formation. However, constitutively active mutants of another Rho target, the formin homology protein mDia1 (Watanabe, N., T. Kato, A. Fujita, T. Ishizaki, and S. Narumiya. 1999. Nat. Cell Biol. 1:136-143), were sufficient to restore force-induced focal contact formation in C3 transferase-treated cells. Force-induced formation of the focal contacts still occurred in cells subjected to myosin II and ROCK inhibition. Thus, as long as mDia1 is active, external tension force bypasses the requirement for ROCK-mediated myosin II contractility in the induction of focal contacts. Our experiments show that integrin-containing focal complexes behave as individual mechanosensors exhibiting directional assembly in response to local force.
Figures
Figure 3
Force-induced focal contact formation as revealed by GFP–paxillin fluorescence. (A and C) GFP–paxillin distribution at the leading edge of serum-starved SV-80 cell 2 min before (A) and 1.5 min after (C) development of the pulling force. (B) Phase image showing the pipette location after the shift. Bar, 10 μm.
Figure 1
Schemes depicting the method of application of external force. The pipette, covered either with poly-
l
-lysine or fibronectin, was bent against the coverslip and moved along the chosen lamellipodium as indicated by the yellow arrow (A). The cross sections through the lamellipodium are shown before (B) and after (C) pipette shift. Tension force applied to a focal complex at the cell edge is represented by the red arrow (C).
Figure 2
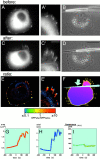
Local formation of focal contacts in response to the application of external force. GFP–vinculin-transfected SV-80 cells incubated in serum-free medium are shown before (A–B) and after (C–D) application of pulling force produced by micropipette shift. (A, A′, C, and C′) GFP fluorescence showing the distribution of vinculin; (B and D) phase image of the same cell. The photographs were taken 2 min before pipette shift (A–B), immediately after the shift (D), and 3 min 37 s after the shift (C and C′). A′ and C′ represent higher magnifications of upper right parts of images A and C, respectively. (E) Image representing pixel-to-pixel ratio between the intensity of the GFP fluorescence 5 min after the pulling and 20 s before pulling. E′ represents high magnification of the upper right part of the image E. The values of the ratio between intensities of after and before images are represented by pseudocolors, as indicated in the spectrum scale shown below E and E′. Thus, red indicates newly formed parts of focal contacts; yellow, stationary parts; and blue, the parts that disappeared. (F) Ratio between the total intensity of individual focal contacts after and before pulling. For each focal contact, the average of the three maximal total intensity values during the 4 min after the pulling was divided by the average of the three maximal values registered during last 5 min before pulling. Positions of the adhesions are indicated by the yellow dots; and the ratio values, by the yellow numbers nearby. The pipette tip is represented by a blue cone; and the direction of the pulling force, by the blue arrow. (G–I) Typical graphs illustrating individual focal adhesion growth. Changes in the focal adhesion area (G), focal adhesion axial ratio (H), and the fluorescence per pixel of the focal contact (I) are shown. Bars: (A, B, C, D, and E; shown in D) 20 μm; (A′, C′, E′; shown in C′) 5 μm.
Figure 4
Force-induced recruitment of GFP–vinculin is accompanied by growth of focal contacts as revealed by IRM observations. GFP–vinculin (A and D) and IRM (B and E) images essentially overlap each other both 7 min before (A and B) and 9 min after (D and E) pulling. Phase image after pulling is shown in the middle (C). Bars: (C) 20 μm; (A, B, D, and E; shown in E) 5 μm.
Figure 6
Function of actin cytoskeleton in the force-induced growth of focal contacts. To probe the role of actin cytoskeleton integrity, SV-80 cells were pretreated with 5 μM latrunculin A (Lat A) (A–B′); to block actomyosin-driven contractility, the cells were pretreated with 30 mM BDM (C–D′) or cotransfected with nonmuscle caldesmon (E–F′). Positions of the pipette tips, immediately after shift, are indicated by blue drawings. B′, D′ and F′ represent enlarged parts of B, D, and F, respectively. Images labeled before and after were taken 1–8 min before and 3–7 min after the pipette shift, respectively. Bars: (B, D, and F) 20 μm; (B′, D′, F′) 5 μm. Note that latrunculin A prevented formation of focal adhesions, whereas neither BDM nor caldesmon transfection interfered with this process.
Figure 5
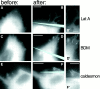
Covering of the substrate with fibronectin is essential for force-induced growth of focal contacts. GFP–vinculin-transfected SV-80 cells plated on poly-
l
-lysine in serum-free medium do not respond to pulling with a fibronectin-coated pipette by formation of focal contacts (D–F), whereas cells plated on substrate precoated with poly-
l
-lysine and then coated with fibronectin (fibronectina) produce normal focal contacts (A–C). Under standard conditions (fibronectinb), cells were plated in serum-containing medium and then serum starved. Pulling of these cells with a poly-
l
-lysine–coated pipette still produces growth of focal contacts (G–I). The positions of pipette immediately after shift are indicated in B, E, and H. Images labeled before and after were taken 1–10 min before and 3–7 min after pipette shift. Bars: (B, E, and H) 10 μm; (C, F, and I) 5 μm. In each before–after pair, the pictures were taken with the same magnification.
Figure 7
Reversible suppression of cell contractility by BDM and Y-27632. SV-80 cells growing on the silicon rubber substrate and producing wrinkles (A and F) were treated with either 30 mM actomyosin ATPase inhibitor BDM (top) or 10 μM ROCK inhibitor Y-27632 (bottom). Images of BDM-treated cells were taken at 2.5 (B), 5 (C), and 9.5 min (D) after drug addition; the drug was washed off after 30 min incubation, and the last image (E) was taken at 45 min after washing. Images of the cell treated with Y-27632 were captured at 2.5 (G), 6 (H), and 9.5 min (I) after drug addition; the drug was washed off after 30 min incubation, and the image (J) was taken 50 min later. Bar, 20 μm.
Figure 8
Involvement of Rho, ROCK, and mDia1 in the force-induced formation of focal contacts. SV-80 cells were cotransfected with GFP–vinculin and C3 toxin (A–C′) or with GFP–vinculin, C3 toxin, and constitutively active mutants of mDia1, mDia1ΔN1 (D–F′) or mDia1ΔN3 (G–I′). Alternatively, the cells transfected with GFP–vinculin were treated with 10 μM ROCK inhibitor Y-27632 before application of force (J–L′). GFP–vinculin distributions in transfected cells several minutes before pipette shift are shown (A, D, G, and J). Positions of the pipettes, immediately after shift, are shown in phase images (B, E, H, and K). GFP–vinculin distributions in the cells 3–7 min after pipette shift are shown (C, F, I, and L). C′, F′, I′, and L′ represent enlarged parts of cells shown in C, F, I, and L, respectively. Newly formed focal contacts are indicated by arrows. Bars, 10 μm.
Figure 9
Scheme depicting involvement of tension force generation in the process of ROCK- and mDia1-dependent formation of focal contacts. Rho induces formation of the focal contacts by activation of two essential pathways, ROCK dependent and mDia1 dependent. The main function of the ROCK-dependent pathway is to activate myosin II–driven cell contractility due to direct or indirect (via myosin light chain phosphatase [MLCP]) effects on the phosphorylation of myosin light chain (yellow crescent). This pathway can be bypassed if the tension force is applied externally. mDia1-dependent pathway includes activation of actin polymerization via interactions with profilin, or possibly other effects on the organization of actin network and microtubules. If mDia1 is active, application of tension force to the focal complexes triggers the transition of these structures into focal contacts and growth of the focal contacts.
Similar articles
- Assembly and mechanosensory function of focal adhesions: experiments and models.
Bershadsky AD, Ballestrem C, Carramusa L, Zilberman Y, Gilquin B, Khochbin S, Alexandrova AY, Verkhovsky AB, Shemesh T, Kozlov MM. Bershadsky AD, et al. Eur J Cell Biol. 2006 Apr;85(3-4):165-73. doi: 10.1016/j.ejcb.2005.11.001. Epub 2005 Dec 19. Eur J Cell Biol. 2006. PMID: 16360240 Review. - ROCK and mDia1 antagonize in Rho-dependent Rac activation in Swiss 3T3 fibroblasts.
Tsuji T, Ishizaki T, Okamoto M, Higashida C, Kimura K, Furuyashiki T, Arakawa Y, Birge RB, Nakamoto T, Hirai H, Narumiya S. Tsuji T, et al. J Cell Biol. 2002 May 27;157(5):819-30. doi: 10.1083/jcb.200112107. Epub 2002 May 20. J Cell Biol. 2002. PMID: 12021256 Free PMC article. - Contractility modulates cell adhesion strengthening through focal adhesion kinase and assembly of vinculin-containing focal adhesions.
Dumbauld DW, Shin H, Gallant ND, Michael KE, Radhakrishna H, García AJ. Dumbauld DW, et al. J Cell Physiol. 2010 Jun;223(3):746-56. doi: 10.1002/jcp.22084. J Cell Physiol. 2010. PMID: 20205236 Free PMC article. - Effects of substrate stiffness and actomyosin contractility on coupling between force transmission and vinculin-paxillin recruitment at single focal adhesions.
Zhou DW, Lee TT, Weng S, Fu J, García AJ. Zhou DW, et al. Mol Biol Cell. 2017 Jul 7;28(14):1901-1911. doi: 10.1091/mbc.E17-02-0116. Epub 2017 May 3. Mol Biol Cell. 2017. PMID: 28468976 Free PMC article. - Assembly and mechanosensory function of focal contacts.
Geiger B, Bershadsky A. Geiger B, et al. Curr Opin Cell Biol. 2001 Oct;13(5):584-92. doi: 10.1016/s0955-0674(00)00255-6. Curr Opin Cell Biol. 2001. PMID: 11544027 Review.
Cited by
- Directional cues in the tumor microenvironment due to cell contraction against aligned collagen fibers.
Szulczewski JM, Inman DR, Proestaki M, Notbohm J, Burkel BM, Ponik SM. Szulczewski JM, et al. Acta Biomater. 2021 Jul 15;129:96-109. doi: 10.1016/j.actbio.2021.04.053. Epub 2021 May 7. Acta Biomater. 2021. PMID: 33965625 Free PMC article. - Inverted formin 2 in focal adhesions promotes dorsal stress fiber and fibrillar adhesion formation to drive extracellular matrix assembly.
Skau CT, Plotnikov SV, Doyle AD, Waterman CM. Skau CT, et al. Proc Natl Acad Sci U S A. 2015 May 12;112(19):E2447-56. doi: 10.1073/pnas.1505035112. Epub 2015 Apr 27. Proc Natl Acad Sci U S A. 2015. PMID: 25918420 Free PMC article. Retracted. - Nanopatterning reveals an ECM area threshold for focal adhesion assembly and force transmission that is regulated by integrin activation and cytoskeleton tension.
Coyer SR, Singh A, Dumbauld DW, Calderwood DA, Craig SW, Delamarche E, García AJ. Coyer SR, et al. J Cell Sci. 2012 Nov 1;125(Pt 21):5110-23. doi: 10.1242/jcs.108035. Epub 2012 Aug 16. J Cell Sci. 2012. PMID: 22899715 Free PMC article. - Nuclear-cytoskeletal linkages facilitate cross talk between the nucleus and intercellular adhesions.
Stewart RM, Zubek AE, Rosowski KA, Schreiner SM, Horsley V, King MC. Stewart RM, et al. J Cell Biol. 2015 May 11;209(3):403-18. doi: 10.1083/jcb.201502024. J Cell Biol. 2015. PMID: 25963820 Free PMC article. - Tension is required but not sufficient for focal adhesion maturation without a stress fiber template.
Oakes PW, Beckham Y, Stricker J, Gardel ML. Oakes PW, et al. J Cell Biol. 2012 Feb 6;196(3):363-74. doi: 10.1083/jcb.201107042. Epub 2012 Jan 30. J Cell Biol. 2012. PMID: 22291038 Free PMC article.
References
- Abercrombie M., Dunn G.A. Adhesions of fibroblasts to substratum during contact inhibition observed by interference reflection microscopy. Exp. Cell Res. 1975;92:57–62. - PubMed
- Aktories K., Hall A. Botulinum ADP-ribosyltransferase C3a new tool to study low molecular weight GTP-binding proteins. Trends Pharmacol. Sci. 1989;10:415–418. - PubMed
- Amano M., Chihara K., Kimura K., Fukata Y., Nakamura N., Matsuura Y., Kaibuchi K. Formation of actin stress fibers and focal adhesions enhanced by Rho- kinase. Science. 1997;275:1308–1311. - PubMed
- Ayscough K. Use of latrunculin-A, an actin monomer-binding drug. Methods Enzymol. 1998;298:18–25. - PubMed
- Balaban N.Q., Schwarz U.S., Riveline D., Goichberg P., Tzur G., Sabanay I., Mahalu D., Safran S., Bershadsky A., Addadi L., Geiger B. Force and focal adhesion assemblya close relationship studied using elastic micro-patterned substrates. Nat. Cell Biol. 2001;3:466–472. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous