Xenopus FRS2 is involved in early embryogenesis in cooperation with the Src family kinase Laloo - PubMed (original) (raw)
Xenopus FRS2 is involved in early embryogenesis in cooperation with the Src family kinase Laloo
M Kusakabe et al. EMBO Rep. 2001 Aug.
Abstract
FRS2 has been identified in mammalian cells as a protein that is tyrosine phosphorylated and binds to Grb2 and Shp2 in response to fibroblast growth factor (FGF) or nerve growth factor (NGF) stimulation. But neither its existence in other vertebrate classes or invertebrates nor its function during embryonic development has been defined. Here we have identified and characterized a Xenopus homolog of FRS2 (xFRS2). xFRS2 is tyrosine phosphorylated in early embryos, and overexpression of an unphosphorylatable form of xFRS2 interferes with FGF-dependent mesoderm formation. The Src family kinase Laloo, which was shown to function in FGF signaling during early Xenopus development, binds to xFRS2 and promotes tyrosine phosphorylation of xFRS2. Moreover, xFRS2 and Laloo are shown to bind to Xenopus FGF receptor 1. These results suggest that xFRS2 plays an important role in FGF signaling in cooperation with Laloo during embryonic development.
Figures
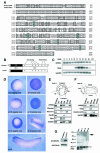
Fig. 1. Cloning and expression of Xenopus FRS2. (A) Alignment of the deduced amino acid sequences of Xenopus FRS2 and human FRS2α. Residues that are identical are boxed. Dashes denote gaps. Underlines indicate P-X-X-P sequences. Six conserved tyrosines are shaded blue. (B) The schematic structures of xFRS2 and human FRS2α. (C) xFRS2 mRNA is present during Xenopus early embryogenesis. The total RNA isolated from indicated stages was subjected to RT–PCR. Xenopus embryonic ornithine decarboxylase (XeODC) is a loading control. Xbra was also examined. No signal was observed in the absence of reverse transcriptase (–RT). (D) Whole-mount in situ hybridization for xFRS2. (E) xFRS2 is expressed in the mesoderm and ectoderm regions at stage 11. Dorsal mesoderm (DM), ventral mesoderm (VM), endoderm (Endo) and ectoderm (Ecto) regions were dissected from ten embryos (stage 11) as shown. Each was processed for RT–PCR. The dorsal mesoderm marker Chordin, the endoderm marker Xsox17α, the ventral mesoderm marker Xwnt8 and the pan-mesodermal marker Xbra were also analyzed. (F) xFRS2 is expressed in the head and dorsal structures at stage 24. Head, dorsal and ventral regions were dissected from five embryos as shown. Each was processed for RT–PCR. The forebrain marker Otx2 and the somitic muscle marker muscle actin were also analyzed. (G) Endogenous xFRS2 is tyrosine phosphorylated. Myc-tagged xFRS2 mRNA was injected and the injected embryos were extracted at stage 11. The extract then was subjected to precipitation with p13_suc1_-agarose beads. Protein G beads were used as a control. The precipitates were detected by anti-myc antibody (left). Extracts of embryos from stage 9 to 26 were subjected to precipitation with p13_suc1_-agarose beads and the precipitates were analyzed with anti-phosphotyrosine antibody (right). A black arrow indicates xFRS2 and a white arrowhead Cdc2. The DDBJ/EMBL/GenBank accession number for xFRS2 is AB064525.
Fig. 2. Involvement of xFRS2 in mesoderm patterning. (A) xFRS2 induces mesodermal markers in animal caps. Wild-type xFRS2 mRNA (100 pg, upper panel; 1 ng, lower panel) was injected into animal poles of two-cell stage embryos. At stage 8, the animal caps were dissected and harvested at midgastrula (upper) or tailbud stages (lower). Total RNA of caps was analyzed by RT–PCR. EF-1α is a loading control. RNA from whole embryos (indicated as embryo) provides a positive control. The concentration of FGF is 50 ng/ml. (B) xFRS2-6F inhibits the induction of mesodermal markers by FGF in animal caps. Indicated amounts of WT (wild type) xFRS2 mRNA or xFRS2-6F mRNA were injected. Animal caps were dissected and cultured without or with FGF (50 ng/ml). Caps were harvested at the midgastrula stage and subjected to RT–PCR. (C) An inhibitory effect of xFRS2-6F on FGF-dependent induction of mesoderm markers is rescued by WT xFRS2. xFRS2-6F mRNA (400 pg) was co-injected with or without WT xFRS2 mRNA (100 pg). Animal caps were cultured in the presence or absence of FGF (50 ng/ml) and subjected to RT–PCR. (D) Expression of mesodermal markers in whole embryos is inhibited by xFRS2-6F. WT xFRS2 mRNA or xFRS2-6F mRNA (400 pg or 2 ng) was injected into the marginal zones of four-cell stage embryos. Injected embryos were collected at stage 11 and subjected to RT–PCR. (E) xFRS2-6F inhibits mesodermal patterning in whole embryos. Indicated amounts of mRNAs were injected into the marginal zones of four-cell stage embryos and cultured until the tadpole stage. Top panel, control embryos; middle, embryos injected with 400 pg of xFRS-6F mRNA (61% with trunk and tail truncations; n = 93) and embryos injected with 2 ng of xFRS2-6F mRNA (73% with trunk and tail truncations; n = 55); bottom, embryos injected with 400 pg of xFRS2-6F mRNA and 4 pg of active Ras (human H-Ras V12) mRNA (17% with trunk and tail truncations; n = 57).
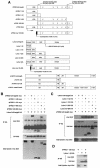
Fig. 3. Interaction of xFRS2 with the Src family kinase Laloo. (A) Laloo enhances tyrosine phosphorylation of xFRS2. Effect of co-injection of xFGFR1 or Laloo Y492F (an active form) on tyrosine phosphorylation of xFRS2 was analyzed. Indicated sets of mRNA were injected at animal poles of 2-cell stage embryos and animal caps were dissected. The lysates of caps were immunoprecipitated (IP) with anti-myc antibody and immunoblotted with anti-phosphotyrosine antibody (upper) or anti-myc antibody (lower). (B) xFGFR1 and Laloo Y492F cooperate to induce tyrosine phosphorylation of xFRS2. The experiments were performed as described in (A). (C) xFRS2 binds to Laloo. Lysates of caps co-injected with the indicated combinations of mRNA encoding myc-tagged xFRS2 or HA-tagged Laloo were immunoprecipitated with anti-HA antibody. Co-immunoprecipitated xFRS2 was detected by blotting with anti-myc antibody (top). The amounts of the precipitated Laloo (middle) and the expression level of xFRS2 (bottom) were also determined. (D) Xbra induction by Laloo Y492F is dependent on xFRS2. Indicated combinations of mRNA encoding Laloo Y492F, WT xFRS2, or xFRS2-6F were co-injected into animal poles of 2-cell stage embryos. The animal cap assay and the RT–PCR analysis were performed as in Figure 2.
Fig. 4. Physical interactions of xFRS2, Laloo and xFGFR1. (A) Schematic representation of full-length and truncated forms of xFRS2, Laloo and xFGFR1. ED, extracellular domain; TM, transmembrane domain; JM, juxtamembrane domain. (B) HA-tagged full-length Laloo was transiently co-expressed with myc-tagged full-length or truncated xFRS2 in C2C12 cells. Lysates of cells were immunoprecipitated (IP) with anti-HA antibody and co-immunoprecipitated full-length or truncated xFRS2 was detected by immunoblotting with anti-myc antibody (top). Comparable amounts of Laloo were immunoprecipitated in each lane (middle). The expression level of full-length or truncated xFRS2 was similar (bottom). (C) Binding of myc-tagged full-length xFRS2 to HA-tagged full-length or truncated Laloo. (D) Binding of myc-tagged xFRS2 (1–128) to HA-tagged Laloo (1–52). (E) Binding of HA-tagged Laloo to myc-tagged full-length or mutated xFRS2. (F) Binding of HA-tagged xFRS2 to myc-tagged full-length or mutated Laloo. (G) Binding of myc-tagged full-length xFRS2 to HA-tagged full-length or truncated xFGFR1. (H) Binding of HA-tagged full-length xFGFR1 to myc-tagged full-length or truncated xFRS2. (I) Binding of HA-tagged full-length Laloo to myc-tagged full-length or truncated xFGFR1. (J) Binding of myc-tagged full-length xFGFR1 to HA-tagged full-length or truncated Laloo. (K) A hypothetical model for the interactions among xFRS2, Laloo and xFGFR1.
Fig. 4. Physical interactions of xFRS2, Laloo and xFGFR1. (A) Schematic representation of full-length and truncated forms of xFRS2, Laloo and xFGFR1. ED, extracellular domain; TM, transmembrane domain; JM, juxtamembrane domain. (B) HA-tagged full-length Laloo was transiently co-expressed with myc-tagged full-length or truncated xFRS2 in C2C12 cells. Lysates of cells were immunoprecipitated (IP) with anti-HA antibody and co-immunoprecipitated full-length or truncated xFRS2 was detected by immunoblotting with anti-myc antibody (top). Comparable amounts of Laloo were immunoprecipitated in each lane (middle). The expression level of full-length or truncated xFRS2 was similar (bottom). (C) Binding of myc-tagged full-length xFRS2 to HA-tagged full-length or truncated Laloo. (D) Binding of myc-tagged xFRS2 (1–128) to HA-tagged Laloo (1–52). (E) Binding of HA-tagged Laloo to myc-tagged full-length or mutated xFRS2. (F) Binding of HA-tagged xFRS2 to myc-tagged full-length or mutated Laloo. (G) Binding of myc-tagged full-length xFRS2 to HA-tagged full-length or truncated xFGFR1. (H) Binding of HA-tagged full-length xFGFR1 to myc-tagged full-length or truncated xFRS2. (I) Binding of HA-tagged full-length Laloo to myc-tagged full-length or truncated xFGFR1. (J) Binding of myc-tagged full-length xFGFR1 to HA-tagged full-length or truncated Laloo. (K) A hypothetical model for the interactions among xFRS2, Laloo and xFGFR1.
Similar articles
- SNT-1/FRS2alpha physically interacts with Laloo and mediates mesoderm induction by fibroblast growth factor.
Hama J, Xu H, Goldfarb M, Weinstein DC. Hama J, et al. Mech Dev. 2001 Dec;109(2):195-204. doi: 10.1016/s0925-4773(01)00524-x. Mech Dev. 2001. PMID: 11731233 Free PMC article. - Docking protein SNT1 is a critical mediator of fibroblast growth factor signaling during Xenopus embryonic development.
Akagi K, Kyun Park E, Mood K, Daar IO. Akagi K, et al. Dev Dyn. 2002 Mar;223(2):216-28. doi: 10.1002/dvdy.10048. Dev Dyn. 2002. PMID: 11836786 - FRS2 proteins recruit intracellular signaling pathways by binding to diverse targets on fibroblast growth factor and nerve growth factor receptors.
Ong SH, Guy GR, Hadari YR, Laks S, Gotoh N, Schlessinger J, Lax I. Ong SH, et al. Mol Cell Biol. 2000 Feb;20(3):979-89. doi: 10.1128/MCB.20.3.979-989.2000. Mol Cell Biol. 2000. PMID: 10629055 Free PMC article. - Regulation of growth factor signaling by FRS2 family docking/scaffold adaptor proteins.
Gotoh N. Gotoh N. Cancer Sci. 2008 Jul;99(7):1319-25. doi: 10.1111/j.1349-7006.2008.00840.x. Epub 2008 Apr 29. Cancer Sci. 2008. PMID: 18452557 Free PMC article. Review. - Exploring the Structural and Functional Diversity among FGF Signals: A Comparative Study of Human, Mouse, and Xenopus FGF Ligands in Embryonic Development and Cancer Pathogenesis.
Goutam RS, Kumar V, Lee U, Kim J. Goutam RS, et al. Int J Mol Sci. 2023 Apr 20;24(8):7556. doi: 10.3390/ijms24087556. Int J Mol Sci. 2023. PMID: 37108717 Free PMC article. Review.
Cited by
- Requirement of the MEK5-ERK5 pathway for neural differentiation in Xenopus embryonic development.
Nishimoto S, Kusakabe M, Nishida E. Nishimoto S, et al. EMBO Rep. 2005 Nov;6(11):1064-9. doi: 10.1038/sj.embor.7400515. Epub 2005 Sep 23. EMBO Rep. 2005. PMID: 16179948 Free PMC article. - Regulation of gene expression downstream of a novel Fgf/Erk pathway during Xenopus development.
Cowell LM, King M, West H, Broadsmith M, Genever P, Pownall ME, Isaacs HV. Cowell LM, et al. PLoS One. 2023 Oct 19;18(10):e0286040. doi: 10.1371/journal.pone.0286040. eCollection 2023. PLoS One. 2023. PMID: 37856433 Free PMC article. - The polarity-inducing kinase Par-1 controls Xenopus gastrulation in cooperation with 14-3-3 and aPKC.
Kusakabe M, Nishida E. Kusakabe M, et al. EMBO J. 2004 Oct 27;23(21):4190-201. doi: 10.1038/sj.emboj.7600381. Epub 2004 Sep 2. EMBO J. 2004. PMID: 15343271 Free PMC article. - Generation of FGF reporter transgenic zebrafish and their utility in chemical screens.
Molina GA, Watkins SC, Tsang M. Molina GA, et al. BMC Dev Biol. 2007 Jun 6;7:62. doi: 10.1186/1471-213X-7-62. BMC Dev Biol. 2007. PMID: 17553162 Free PMC article. - Vertebrate Ctr1 coordinates morphogenesis and progenitor cell fate and regulates embryonic stem cell differentiation.
Haremaki T, Fraser ST, Kuo YM, Baron MH, Weinstein DC. Haremaki T, et al. Proc Natl Acad Sci U S A. 2007 Jul 17;104(29):12029-34. doi: 10.1073/pnas.0701413104. Epub 2007 Jul 9. Proc Natl Acad Sci U S A. 2007. PMID: 17620605 Free PMC article.
References
- Amaya E., Musci, T.J. and Kirschner, M.W. (1991) Expression of a dominant negative mutant of the FGF receptor disrupts mesoderm formation in Xenopus embryos. Cell, 66, 257–270. - PubMed
- Basilico C. and Moscatelli, D. (1992) The FGF family of growth factors and oncogenes. Adv. Cancer. Res., 59, 115–165. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous