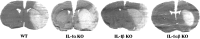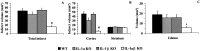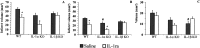Role of IL-1alpha and IL-1beta in ischemic brain damage - PubMed (original) (raw)
Role of IL-1alpha and IL-1beta in ischemic brain damage
H Boutin et al. J Neurosci. 2001.
Erratum in
- J Neurosci 2001 Sep 1;21(17):1a
Abstract
The cytokine interleukin-1 (IL-1) has been strongly implicated in the pathogenesis of ischemic brain damage. Evidence to date suggests that the major form of IL-1 contributing to ischemic injury is IL-1beta rather than IL-1alpha, but this has not been tested directly. The objective of the present study was to compare the effects of transient cerebral ischemia [30 min middle cerebral artery occlusion (MCAO)] on neuronal injury in wild-type (WT) mice and in IL-1alpha, IL-1beta, or both IL-1alpha and IL-1beta knock-out (KO) mice. Mice lacking both forms of IL-1 exhibited dramatically reduced ischemic infarct volumes compared with wild type (total volume, 70%; cortex, 87% reduction). Ischemic damage compared with WT mice was not significantly altered in mice lacking either IL-1alpha or IL-1beta alone. IL-1beta mRNA, but not IL-1alpha or the IL-1 type 1 receptor, was strongly induced by MCAO in WT and IL-1alpha KO mice. Administration (intracerebroventricularly) of recombinant IL-1 receptor antagonist significantly reduced infarct volume in WT (-32%) and IL-1alpha KO (-48%) mice, but had no effect on injury in IL-1beta or IL-1alpha/beta KO mice. These data confirm that IL-1 plays a major role in ischemic brain injury. They also show that chronic deletion of IL-1alpha or IL-1beta fails to influence brain damage, probably because of compensatory changes in the IL-1 system in IL-1alpha KO mice and changes in IL-1-independent mediators of neuronal death in IL-1beta KO mice.
Figures
Fig. 1.
Representative coronal brain sections (20 μm) of WT, IL-1α KO, IL-1β KO, and IL-1αβ KO mice 24 hr after 30 min of middle cerebral artery occlusion.
Fig. 2.
Effect of 30 min middle cerebral artery occlusion on infarct volume (A, total; B, cortex and striatum) and edema (C) in WT, IL-1α KO, IL-1β KO, and IL-1αβ KO mice (n = 9 per group). Volumes are expressed in cubic millimeters (mean ± SEM). *Significantly different to WT, and #significantly different to WT, IL-1α KO, and IL-1β KO mice, respectively (p < 0.05, ANOVA followed by Scheffe_post hoc_ test).
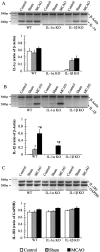
Fig. 3.
Representative PCR gels, with corresponding image analysis quantification for IL-1α (A), IL-1β (B), and IL-1RI (C) in ipsilateral cortices in WT (control, n = 4; sham,n = 3; MCAO, n = 4), IL-1α KO (control, n = 3; sham, n = 3; MCAO, n = 4), and IL-1β KO mice (control,n = 3; sham, n = 4; MCAO,n = 5). Data are expressed as the ratio of the gene of interest to the relevant control gene (β-actin for IL-1α and IL-1β and GAPDH for IL-1RI; mean ± SEM). Significantly different from control (*) and sham-operated (#) groups (p < 0.05; Mann–Whitney_U_ test).
Fig. 4.
Comparison of total (A) and cortical infarct (B) volumes, corrected for edema (expressed in cubic millimeters; mean ± SEM) and edema (C) between saline and IL-1ra treatments, in WT, IL-1α KO, and IL-1β KO mice (WT/saline, n = 13; WT/IL1-ra,n = 10; IL-1α KO/saline, n = 10; IL-1α KO/IL-1ra, n = 10; IL-1β KO/saline,n = 12; IL-1β KO/IL-1ra, n = 11). *Indicates significant difference from saline-treated group. #Indicates a significant difference from WT receiving identical treatment (p < 0.05, ANOVA followed by Scheffe post hoc test).
Similar articles
- Interleukin-1 influences ischemic brain damage in the mouse independently of the interleukin-1 type I receptor.
Touzani O, Boutin H, LeFeuvre R, Parker L, Miller A, Luheshi G, Rothwell N. Touzani O, et al. J Neurosci. 2002 Jan 1;22(1):38-43. doi: 10.1523/JNEUROSCI.22-01-00038.2002. J Neurosci. 2002. PMID: 11756486 Free PMC article. - Early increase in mRNA levels of pro-inflammatory cytokines and their interactions in the mouse hippocampus after transient global ischemia.
Zhu Y, Saito K, Murakami Y, Asano M, Iwakura Y, Seishima M. Zhu Y, et al. Neurosci Lett. 2006 Jan 30;393(2-3):122-6. doi: 10.1016/j.neulet.2005.08.072. Epub 2005 Dec 13. Neurosci Lett. 2006. PMID: 16356636 - Production of mice deficient in genes for interleukin (IL)-1alpha, IL-1beta, IL-1alpha/beta, and IL-1 receptor antagonist shows that IL-1beta is crucial in turpentine-induced fever development and glucocorticoid secretion.
Horai R, Asano M, Sudo K, Kanuka H, Suzuki M, Nishihara M, Takahashi M, Iwakura Y. Horai R, et al. J Exp Med. 1998 May 4;187(9):1463-75. doi: 10.1084/jem.187.9.1463. J Exp Med. 1998. PMID: 9565638 Free PMC article. - Involvement of cytokine production in pathogenesis of transient cerebral ischemic damage.
Yamasaki Y, Itoyama Y, Kogure K. Yamasaki Y, et al. Keio J Med. 1996 Sep;45(3):225-9. doi: 10.2302/kjm.45.225. Keio J Med. 1996. PMID: 8897765 Review. - Interleukin 1α: a comprehensive review on the role of IL-1α in the pathogenesis and treatment of autoimmune and inflammatory diseases.
Cavalli G, Colafrancesco S, Emmi G, Imazio M, Lopalco G, Maggio MC, Sota J, Dinarello CA. Cavalli G, et al. Autoimmun Rev. 2021 Mar;20(3):102763. doi: 10.1016/j.autrev.2021.102763. Epub 2021 Jan 20. Autoimmun Rev. 2021. PMID: 33482337 Review.
Cited by
- LncRNA SNHG4 Attenuates Inflammatory Responses by Sponging miR-449c-5p and Up-Regulating STAT6 in Microglial During Cerebral Ischemia-Reperfusion Injury.
Zhang S, Sun WC, Liang ZD, Yin XR, Ji ZR, Chen XH, Wei MJ, Pei L. Zhang S, et al. Drug Des Devel Ther. 2020 Sep 11;14:3683-3695. doi: 10.2147/DDDT.S245445. eCollection 2020. Drug Des Devel Ther. 2020. PMID: 32982175 Free PMC article. - Neuroinflammation in Post-Ischemic Neurodegeneration of the Brain: Friend, Foe, or Both?
Pluta R, Januszewski S, Czuczwar SJ. Pluta R, et al. Int J Mol Sci. 2021 Apr 23;22(9):4405. doi: 10.3390/ijms22094405. Int J Mol Sci. 2021. PMID: 33922467 Free PMC article. Review. - Post-ischemic inflammation in the brain.
Shichita T, Sakaguchi R, Suzuki M, Yoshimura A. Shichita T, et al. Front Immunol. 2012 May 31;3:132. doi: 10.3389/fimmu.2012.00132. eCollection 2012. Front Immunol. 2012. PMID: 22833743 Free PMC article. - System x(c)- activity and astrocytes are necessary for interleukin-1 beta-mediated hypoxic neuronal injury.
Fogal B, Li J, Lobner D, McCullough LD, Hewett SJ. Fogal B, et al. J Neurosci. 2007 Sep 19;27(38):10094-105. doi: 10.1523/JNEUROSCI.2459-07.2007. J Neurosci. 2007. PMID: 17881516 Free PMC article. - Acute hypoxia activates the neuroimmune system, which diabetes exacerbates.
Johnson DR, O'Connor JC, Hartman ME, Tapping RI, Freund GG. Johnson DR, et al. J Neurosci. 2007 Jan 31;27(5):1161-6. doi: 10.1523/JNEUROSCI.4560-06.2007. J Neurosci. 2007. PMID: 17267571 Free PMC article.
References
- Akita K, Ohtsuki T, Nukada Y, Tanimoto T, Namba M, Okura T, Takakura-Yamamoto R, Torigoe K, Gu Y, Su MS, Fujii M, Satoh-Itoh M, Yamamoto K, Kohno K, Ikeda M, Kurimoto M. Involvement of caspase-1 and caspase-3 in the production and processing of mature human interleukin 18 in monocytic THP.1 cells. J Biol Chem. 1997;272:26595–26603. - PubMed
- Alheim K, Chai Z, Fantuzzi G, Hasanvan H, Malinowsky D, Di Santo E, Ghezzi P, Dinarello CA, Bartfai T. Hyperresponsive febrile reactions to interleukin (IL)-1alpha and IL-1beta, and altered brain cytokine mRNA and serum cytokine levels, in IL-1beta-deficient mice. Proc Natl Acad Sci USA. 1997;94:2681–2686. - PMC - PubMed
- Allan SM, Lawrence CB, Grundy RP, Stroemer RP, Rothwell NJ. Sites and mechanisms of interleukin-1 action. In: Krieglstein J, Oberpichler-Schwenk H, editors. Pharmacology of cerebral ischemia. Medpharm Scientific; Stuttgart: 1998. pp. 395–399.
- Betz AL, Yang GY, Davidson BL. Attenuation of stroke size in rats using an adenoviral vector to induce overexpression of interleukin-1 receptor antagonist in brain. J Cereb Blood Flow Metab. 1995;15:547–551. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials