Spatial shaping of cochlear innervation by temporally regulated neurotrophin expression - PubMed (original) (raw)
Comparative Study
Spatial shaping of cochlear innervation by temporally regulated neurotrophin expression
I Fariñas et al. J Neurosci. 2001.
Abstract
Previous work suggested qualitatively different effects of neurotrophin 3 (NT-3) in cochlear innervation patterning in different null mutants. We now show that all NT-3 null mutants have a similar phenotype and lose all neurons in the basal turn of the cochlea. To understand these longitudinal deficits in neurotrophin mutants, we have compared the development of the deficit in the NT-3 mutant to the spatial-temporal expression patterns of brain-derived neurotrophic factor (BDNF) and NT-3, using lacZ reporters in each gene and with expression of the specific neurotrophin receptors, trkB and trkC. In the NT-3 mutant, almost normal numbers of spiral ganglion neurons form, but fiber outgrowth to the basal turn is eliminated by embryonic day (E) 13.5. Most neurons are lost between E13.5 and E15.5. During the period preceding apoptosis, NT-3 is expressed in supporting cells, whereas BDNF is expressed mainly in hair cells, which become postmitotic in an apical to basal temporal gradient. During the period of neuronal loss, BDNF is absent from the basal cochlea, accounting for the complete loss of basal turn neurons in the NT-3 mutant. The spatial gradients of neuronal loss in these two mutants appear attributable to spatial-temporal gradients of neurotrophin expression. Our immunocytochemical data show equal expression of their receptors, TrkB and TrkC, in spiral sensory neurons and thus do not relate to the basal turn loss. Mice in which NT-3 was replaced by BDNF show a qualitative normal pattern of innervation at E13.5. This suggests that the pattern of expression of neurotrophins rather than their receptors is essential for the spatial loss of spiral sensory neurons in NT-3 null mutants.
Figures
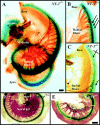
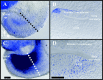
Fig. 1.
Distributions of nerve fibers in the cochlea of_NT-3_ and BDNF heterozygous and homozygous mutant mice at birth. Whole-mounted cochleas from a newborn_NT-3_ homozygous mutant (A, C) and an_NT-3_ heterozygous littermate (B), a BDNF heterozygous mutant (D), and a BDNF homozygous mutant (E) showing the distribution of NT-3(A–C) and of BDNF (D, E) as revealed by β-galactosidase histochemistry and the distribution of nerve fibers as revealed by acetylated tubulin immunocytochemistry. Notice that the cochlea is labeled uniformly throughout its extent by the blue histochemical product. In_NT-3_ null mice, no radial fibers are present in the basal turn (A, C). The only innervation present in this turn is supplied by the few fibers diverted from the middle turn that extend along the longitudinal axis of the cochlea next to inner hair cells (C, crossed arrows). The only outer hair cells in the basal turn approached by afferent fibers were those adjacent to the middle turn (arrow). This contrasts with the presence of numerous radial fibers innervating inner and outer hair cells in the basal turn of an NT-3 heterozygous littermate (B, arrows). The density of radial fiber, as revealed by an increase in the spacing between radial fiber bundles, is reduced in the absence of BDNF(E) when compared with the heterozygous condition (D). This reduction in fiber density is stronger in the apex of BDNF null mutants. Thus, the homogeneous distribution of NT-3 and _BDNF_at birth does not explain the selective loss of basal turn spiral neurons in NT-3 null mutants or the reduced density of radial fibers in the apex of BDNF null mutants.ggl, Ganglion; RF, radial fiber bundles. Scale bars, 0.1 mm.
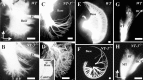
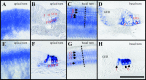
Fig. 2.
Development of cochlear afferent innervation at E12.5 (A, B), E13.5 (C, D,inset), and P0 (E, F), and of central projections at P0 (G, H).Arrows in A show orientation for_A_–F; arrows in_H_ show orientation for the coronal sections of_G_ and H. Outgrowth of afferents (A, B) is labeled with DiI in E12.5 wild-type (A) and NT-3 mutant (B) littermates. Note projection to the basal turn in both cases. At E13.5, the NT-3 mutant (C) lacks radial afferents in the base of the cochlea. In contrast, NT-3 tgBDNF_mutants show a pattern of innervation reminiscent of same age wild-type animals (inset) with an overall reduced density of innervation but a clear innervation of the basal turn (D). Newborn NT-3 mutant mice from a different genetic strain (Ernfors et al., l994) show comparable patterns of innervation (E–F). Compared with the dense radial afferent bundles in the wild types (E), notice the complete absence of radial afferents in the basal turn of mutants (F) and exclusive innervation of inner hair cells. Cochlear afferents to the brainstem in control (G) and in_NT-3 mutant (H) littermates show a topologically restricted projection from the base and the middle turn to the more dorsal and ventral aspects of the ventral cochlear nucleus, respectively. The distinction between the two areas of projection is less pronounced in NT-3 mutants, presumably because the remaining sensory fibers occupy the areas normally innervated by the lost afferents. Comparable coronal section planes are indicated by the presence of olivocochlear efferent fibers (G, H). BT, Basal turn;MT, middle turn; OCB, olivo-cochlear bundle; PC, fibers to the posterior crista;WT, wild type. Scale bars, 100 μm.
Fig. 3.
Developmental expression of _trk_B (A, C, E,F) and _trk_C (B,D, E, G) in developing otic sensory neurons at E11.5 (A, B), E13.5 (C–E), and E15.5 (F, G). The distribution of the trk proteins was revealed using specific primary antibodies (Fariñas et al., 1998) and peroxidase (A, B, F,G), fluorescein (C, E), or rhodamine (D, E) -conjugated secondary antibodies. trkB and trkC are expressed in all otic neurons at early stages (A, B) and in all cochlear neurons until at least E15.5 (F, G), respectively. Fluorescent double labeling at E13.5 for both_trk_B and _trk_C indicates that all cochlear neurons express both trk proteins (C–E). Scale bar (shown in E):A, B, F, G, 100 μm; C_–_E, 25 μm.
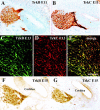
Fig. 4.
Developmental expression of endogenous_BDNF_ (A–D) and_NT-3_ (E–H) as revealed by β-galactosidase histochemistry in whole-mounted cochleas from heterozygous animals with a lacZ reporter gene inserted into the BDNF and NT-3 locus, respectively. Arrows in E indicate orientation. BDNF expression is first detected at E10.5 (A). Expression is strongest in the posteroventral aspect, but it also forms a crest around the anterior and dorsal aspect. The anteroventral aspect adjacent to the forming otic ganglion is notably devoid of BDNF labeling. Interestingly, this area receives the first growing fibers (A). By E11.5 (B),BDNF expression in the posteroventral quadrant has intensified and is related to the growing cochlear duct, the semicircular canal sensory epithelia, and the forming cochleovestibular ganglion (Ggl). Streaks of β-galactosidase-positive cells (*) were seen apparently migrating away from the otocyst and toward the forming ganglion and were even more apparent at E12.5 (C) because of the more distant spacing of the epithelia in the growing otocyst. Interestingly,BDNF expression in the growing cochlea is sharply downregulated at E12.5, except for a faint expression restricted to the growing tip (C). The utricle (U) and saccule (S) show only faint and restricted expression at this stage. At E13.5 (D) and later, the expression remains rather stable in all sensory epithelia, except for a progressive upregulation of BDNF that proceeds toward the base of the cochlea. In contrast to BDNF, expression of NT-3 at E10.5 (E) forms two continuous patches, the primordia of the future utricle and of the future saccule and cochlea. Faint NT-3 expression is also visible in the forming otic ganglion (Ggl), in streaks of cells extending from discrete regions of the otocyst to the otic ganglion (*), and in the endolymphatic duct. By E11.5 (F), the β-galactosidase-positive utricle and saccule + cochlea (S + C) have segregated. By E12.5 (G), the_NT-3-_positive portion comprises only a fraction of the total otic ganglion, and the saccule and cochlea are beginning to segregate. By E13.5 (H), the utricle, saccule, and cochlea are anatomically distinct from each other, and all of them are intensely positive. Note that the ganglion was removed for clarity in H. The gap between the saccule and the cochlea turns into the ductus reuniens (DR). There is some expression of NT-3 in the sensory cristae of the three semicircular canals (anterior, horizontal, posterior).AC, Anterior crista; C, cochlea;ED, endolymphatic duct; Ggl, otic (cochleovestibular) ganglion; HC, horizontal crista;PC, posterior crista. Scale bars: 100 μm.
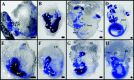
Fig. 5.
Expression of BDNF (A, B) and NT-3 (C, D) in the cochlea at E12.5. Arrows in A indicate orientation, and dashed lines in A,C indicate plane of section for B,D. Note that BDNF expression is very faint and restricted to the apex of the cochlea (A). In contrast, NT-3 expression is more robust, although it is absent from the very apical tip (C). Plastic sections (10 μm thick) of X-gal-reacted cochleas show that BDNF expression in the base is restricted to an area of the cochlear duct that will become the Reissner's membrane (B). In contrast,NT-3 expression is seen throughout the greater epithelial ridge (GER), an area that will become the organ of Corti and the inner spiral sulcus. Scale bars, 100 μm.
Fig. 6.
Expression of NT-3(A–D) and BDNF(E–H) in the cochlea at birth as seen in whole mounts (A, C, E,G) and in 10-μm-thick (F, H) or 2-μm-thick (B, D) plastic sections. Note that in the apical turn (A, B, E,F), neither NT-3 (A, B) nor BDNF expression (E, F) is restricted to hair cells. In fact,NT-3 expression is predominantly seen in supporting cells that also express some BDNF. In contrast, in the basal turn (C, D, G,H), BDNF expression is restricted to hair cells (G, H), whereas _NT-3_is expressed by inner hair cells and their surrounding supporting cells. D, Deiter's cells; GER, greater epithelial ridge; I, inner hair cells; O, outer hair cells; P, Pillar cells. Scale bar, 100 μm.
Fig. 7.
This scheme depicts the dynamics of_BDNF_ (green) and_NT-3_ (pink) expression during cochlear development (left column) and the effects of transgenes and null mutations (right column). Note that at E12.5, BDNF is restricted to the apex of the cochlea, whereas NT-3 is prominently expressed in the base and middle turn, mainly around the three rows of outer hair cells. Sensory neurons (red circles) have become postmitotic in the basal half and extend their fibers. By E14.5, the expression of_BDNF_ has expanded toward the most basal part of the middle turn (mainly in inner hair cells), whereas NT-3_expression has expanded toward the growing apex (mainly in outer hair cells). At E16.5, expression of both BDNF and_NT-3 extends throughout the cochlea longitudinally. However, the basal turn expresses NT-3 around all hair cells and in inner hair cells, whereas the apex has no expression in or around inner hair cells. In contrast, BDNF expression is found in all hair cells as well as faintly in supporting cells in the apex, whereas it is restricted to hair cells in the middle and basal turns. The arrows (Age, Maturation) refer to hair cells only. NT-3 null mutants (right column, middle) have no neurotrophin to support basal turn sensory neurons. Surviving middle turn sensory neurons rearrange their axonal trajectory toward the base. This seems to reflect the progressive upregulation of BDNF in inner hair cells (bottom). In contrast, the effect of BDNF_null mutation becomes apparent later and in the apex. Replacing_NT-3 with BDNF restores the innervation of the basal turn (top). We propose that the longitudinal gradients, which mimic the age gradient of the cochlea in the case of BDNF and the maturation gradient of the cochlea in the case of NT-3, are responsible for the specific reduction of sensory neurons in the base of_NT-3_ null mutants and in the apex of _BDNF_null mutants. IHC's, Inner hair cells;OHC's, outer hair cells.
Similar articles
- Absence of sensory neurons before target innervation in brain-derived neurotrophic factor-, neurotrophin 3-, and TrkC-deficient embryonic mice.
Liebl DJ, Tessarollo L, Palko ME, Parada LF. Liebl DJ, et al. J Neurosci. 1997 Dec 1;17(23):9113-21. doi: 10.1523/JNEUROSCI.17-23-09113.1997. J Neurosci. 1997. PMID: 9364058 Free PMC article. - Effects of neurotrophin and neurotrophin receptor disruption on the afferent inner ear innervation.
Fritzsch B, Silos-Santiago I, Bianchi LM, Farinas I. Fritzsch B, et al. Semin Cell Dev Biol. 1997;8:277-84. Semin Cell Dev Biol. 1997. PMID: 11542690 Review. - The combined effects of trkB and trkC mutations on the innervation of the inner ear.
Fritzsch B, Barbacid M, Silos-Santiago I. Fritzsch B, et al. Int J Dev Neurosci. 1998 Oct;16(6):493-505. doi: 10.1016/s0736-5748(98)00043-4. Int J Dev Neurosci. 1998. PMID: 9881298 - NT-3 replacement with brain-derived neurotrophic factor redirects vestibular nerve fibers to the cochlea.
Tessarollo L, Coppola V, Fritzsch B. Tessarollo L, et al. J Neurosci. 2004 Mar 10;24(10):2575-84. doi: 10.1523/JNEUROSCI.5514-03.2004. J Neurosci. 2004. PMID: 15014133 Free PMC article. - Neurotrophins in the ear: their roles in sensory neuron survival and fiber guidance.
Fritzsch B, Tessarollo L, Coppola E, Reichardt LF. Fritzsch B, et al. Prog Brain Res. 2004;146:265-78. doi: 10.1016/S0079-6123(03)46017-2. Prog Brain Res. 2004. PMID: 14699969 Review.
Cited by
- BDNF profoundly and specifically increases KCNQ4 expression in neurons derived from embryonic stem cells.
Purcell EK, Yang A, Liu L, Velkey JM, Morales MM, Duncan RK. Purcell EK, et al. Stem Cell Res. 2013 Jan;10(1):29-35. doi: 10.1016/j.scr.2012.08.005. Epub 2012 Sep 5. Stem Cell Res. 2013. PMID: 23089626 Free PMC article. - Age-Dependency of Neurite Outgrowth in Postnatal Mouse Cochlear Spiral Ganglion Explants.
Frick C, Fink S, Schmidbauer D, Rousset F, Eickhoff H, Tropitzsch A, Kramer B, Senn P, Glueckert R, Rask-Andersen H, Wiesmüller KH, Löwenheim H, Müller M. Frick C, et al. Brain Sci. 2020 Aug 21;10(9):580. doi: 10.3390/brainsci10090580. Brain Sci. 2020. PMID: 32839381 Free PMC article. - The Three-Dimensional Culture System with Matrigel and Neurotrophic Factors Preserves the Structure and Function of Spiral Ganglion Neuron In Vitro.
Sun G, Liu W, Fan Z, Zhang D, Han Y, Xu L, Qi J, Zhang S, Gao BT, Bai X, Li J, Chai R, Wang H. Sun G, et al. Neural Plast. 2016;2016:4280407. doi: 10.1155/2016/4280407. Epub 2016 Jan 6. Neural Plast. 2016. PMID: 27057364 Free PMC article. - Sphingosine 1-phosphate signaling pathway in inner ear biology. New therapeutic strategies for hearing loss?
Romero-Guevara R, Cencetti F, Donati C, Bruni P. Romero-Guevara R, et al. Front Aging Neurosci. 2015 Apr 23;7:60. doi: 10.3389/fnagi.2015.00060. eCollection 2015. Front Aging Neurosci. 2015. PMID: 25954197 Free PMC article. Review. - Improved Auditory Nerve Survival with Nanoengineered Supraparticles for Neurotrophin Delivery into the Deafened Cochlea.
Wise AK, Tan J, Wang Y, Caruso F, Shepherd RK. Wise AK, et al. PLoS One. 2016 Oct 27;11(10):e0164867. doi: 10.1371/journal.pone.0164867. eCollection 2016. PLoS One. 2016. PMID: 27788219 Free PMC article.
References
- Adam J, Myat A, LeRoux I, Eddison M, Henrique D, Ish-Horowicz D, Lewis J. Cell fate choices and the expression of Notch, Delta and Serrate homologues in the chick inner ear: parallel with Drosophila sense-organ development. Development. 1998;125:4645–4654. - PubMed
- Altman J, Bayer S. Development of the cranial nerve ganglia and related nuclei in the rat. Adv Anat Embryol Cell Biol. 1982;74:1–90. - PubMed
- Bennett JL, Zeiler SR, Jones KR. Patterned expression of BDNF and NT-3 in the retina and anterior segment of the developing mammalian eye. Invest Ophthalmol Vis Sci. 1999;40:2996–3005. - PubMed
- Bianchi LM, Conover JC, Fritzsch B, De Chiara T, Lindsay RM, Yancopoulos GD. Degeneration of vestibular neurons in late embryogenesis of both heterozygous and homozygous BDNF null mutant mice. Development. 1996;122:1965–1973. - PubMed
- Carney PR, Silver J. Studies on cell migration and axon guidance in the developing distal auditory system of the mouse. J Comp Neurol. 1983;215:359–369. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- P 50 DC 00215/DC/NIDCD NIH HHS/United States
- KO1 NS01872/NS/NINDS NIH HHS/United States
- WT_/Wellcome Trust/United Kingdom
- P50 MH048200-100005/MH/NIMH NIH HHS/United States
- 48200/PHS HHS/United States
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials