New EMBO members' review: actin cytoskeleton regulation through modulation of PI(4,5)P(2) rafts - PubMed (original) (raw)
Review
New EMBO members' review: actin cytoskeleton regulation through modulation of PI(4,5)P(2) rafts
P Caroni. EMBO J. 2001.
Abstract
The phosphoinositide lipid PI(4,5)P(2) is now established as a key cofactor in signaling to the actin cytoskeleton and in vesicle trafficking. PI(4,5)P(2) accumulates at membrane rafts and promotes local co-recruitment and activation of specific signaling components at the cell membrane. PI(4,5)P(2) rafts may thus be platforms for local regulation of morphogenetic activity at the cell membrane. Raft PI(4,5)P(2) is regulated by lipid kinases (PI5-kinases) and lipid phosphatases (e.g. synaptojanin). In addition, GAP43-like proteins have recently emerged as a group of PI(4,5)P(2) raft-modulating proteins. These locally abundant proteins accumulate at inner leaflet plasmalemmal rafts where they bind to and co-distribute with PI(4,5)P(2), and promote actin cytoskeleton accumulation and dynamics. In keeping with their proposed role as positive modulators of PI(4,5)P(2) raft function, GAP43-like proteins confer competence for regulated morphogenetic activity on cells that express them. Their function has been investigated extensively in the nervous system, where their expression promotes neurite outgrowth, anatomical plasticity and nerve regeneration. Extrinsic signals and intrinsic factors may thus converge to modulate PI(4,5)P(2) rafts, upstream of regulated activity at the cell surface.
Figures
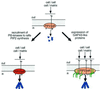
Fig. 1. Pathways of PI(4,5)P2 raft modulation and actin cytoskeleton regulation at rafts. Top: PI(4,5)P2 (red) accumulates at subplasma lemmal rafts. Left: Signals through e.g. Rho-GTPases recruit activated PI5-kinases at rafts leading to enhanced contents of PI(4,5)P2 at rafts (darker red). Right: GAP43-like proteins (green) accumulate at PI(4,5)P2 rafts. By promoting raft assembly, GAP43-like proteins enhance signal strength at PI(4,5)P2 rafts. In both cases, elevated signaling by PI(4,5)P2 at rafts would enhance recruitment and activation of WASP proteins and ERM proteins, to promote actin polymerization and actin filament assembly (blue). Local interactions with extracellular matrix or cell surface components can modulate raft function, e.g. through tyrosine kinase activation, mediating spatial control of actin regulation, and promoting further signal amplification. See text for further details.
Similar articles
- PI(4,5)P2-dependent microdomain assemblies capture microtubules to promote and control leading edge motility.
Golub T, Caroni P. Golub T, et al. J Cell Biol. 2005 Apr 11;169(1):151-65. doi: 10.1083/jcb.200407058. Epub 2005 Apr 4. J Cell Biol. 2005. PMID: 15809307 Free PMC article. - GAP43, MARCKS, and CAP23 modulate PI(4,5)P(2) at plasmalemmal rafts, and regulate cell cortex actin dynamics through a common mechanism.
Laux T, Fukami K, Thelen M, Golub T, Frey D, Caroni P. Laux T, et al. J Cell Biol. 2000 Jun 26;149(7):1455-72. doi: 10.1083/jcb.149.7.1455. J Cell Biol. 2000. PMID: 10871285 Free PMC article. - Spatial control of actin-based motility through plasmalemmal PtdIns(4,5)P2-rich raft assemblies.
Golub T, Pico C. Golub T, et al. Biochem Soc Symp. 2005;(72):119-27. doi: 10.1042/bss0720119. Biochem Soc Symp. 2005. PMID: 15649136 Review. - Temporal proteomics profiling of lipid rafts in CCR6-activated T cells reveals the integration of actin cytoskeleton dynamics.
Lin SL, Chien CW, Han CL, Chen ES, Kao SH, Chen YJ, Liao F. Lin SL, et al. J Proteome Res. 2010 Jan;9(1):283-97. doi: 10.1021/pr9006156. J Proteome Res. 2010. PMID: 19928914 - Tether and trap: regulation of membrane-raft dynamics by actin-binding proteins.
Viola A, Gupta N. Viola A, et al. Nat Rev Immunol. 2007 Nov;7(11):889-96. doi: 10.1038/nri2193. Nat Rev Immunol. 2007. PMID: 17948020 Review.
Cited by
- Invasive cells in animals and plants: searching for LECA machineries in later eukaryotic life.
Vaškovičová K, Žárský V, Rösel D, Nikolič M, Buccione R, Cvrčková F, Brábek J. Vaškovičová K, et al. Biol Direct. 2013 Apr 4;8:8. doi: 10.1186/1745-6150-8-8. Biol Direct. 2013. PMID: 23557484 Free PMC article. Review. - Membrane raft redox signalosomes in endothelial cells.
Zhang C, Li PL. Zhang C, et al. Free Radic Res. 2010 Aug;44(8):831-42. doi: 10.3109/10715762.2010.485994. Free Radic Res. 2010. PMID: 20528560 Free PMC article. Review. - AAV-KLF7 Promotes Descending Propriospinal Neuron Axonal Plasticity after Spinal Cord Injury.
Li WY, Wang Y, Zhai FG, Sun P, Cheng YX, Deng LX, Wang ZY. Li WY, et al. Neural Plast. 2017;2017:1621629. doi: 10.1155/2017/1621629. Epub 2017 Aug 13. Neural Plast. 2017. PMID: 28884027 Free PMC article. - GAP-43 gene expression regulates information storage.
Holahan MR, Honegger KS, Tabatadze N, Routtenberg A. Holahan MR, et al. Learn Mem. 2007 Jun 6;14(6):407-15. doi: 10.1101/lm.581907. Print 2007 Jun. Learn Mem. 2007. PMID: 17554085 Free PMC article. - PI(4,5)P2-dependent microdomain assemblies capture microtubules to promote and control leading edge motility.
Golub T, Caroni P. Golub T, et al. J Cell Biol. 2005 Apr 11;169(1):151-65. doi: 10.1083/jcb.200407058. Epub 2005 Apr 4. J Cell Biol. 2005. PMID: 15809307 Free PMC article.
References
- Aderem A. (1995) The MARCKS family of protein kinase-C substrates. Biochem. Soc. Trans., 23, 587–591. - PubMed
- Aigner L., Arber,S., Kapfhammer,J.P., Laux,T., Schneider,C., Botteri,F., Brenner,H.R. and Caroni,P. (1995) Overexpression of the neural growth-associated protein GAP-43 induces nerve sprouting in the adult nervous system of transgenic mice. Cell, 83, 269–278. - PubMed
- Benowitz L.I. and Routtenberg A. (1997) GAP-43: an intrinsic determinant of neuronal development and plasticity. Trends Neurosci., 20, 84–91. - PubMed
- Bomze H.M., Bulsara,K.R., Iskandar,B.J., Caroni,P. and Skene,P.J.H. (2001) Spinal axon regeneration evoked by replacing two growth cone proteins in adult neurons. Nature Neurosci., 4, 38–43. - PubMed
- Brown D.A. and London,E. (2000) Structure and function of sphingolipid- and cholesterol-rich membrane rafts. J. Biol. Chem., 275, 17221–17224. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous