Germinal center-associated nuclear protein (GANP) has a phosphorylation-dependent DNA-primase activity that is up-regulated in germinal center regions - PubMed (original) (raw)
Germinal center-associated nuclear protein (GANP) has a phosphorylation-dependent DNA-primase activity that is up-regulated in germinal center regions
K Kuwahara et al. Proc Natl Acad Sci U S A. 2001.
Abstract
Antigen stimulation induces a rapid proliferation of B cells for expansion of specific B cell clones and their further differentiation into antibody-producing cells in germinal centers of T-dependent antigen-immunized mice. Previously, we identified a 210-kDa germinal center-associated nuclear protein (GANP) that is up-regulated selectively in germinal centers and carries an MCM-binding domain in the carboxyl-terminal side. In addition, here, we found a region (from 414 to 550 aa) in GANP molecule that is slightly similar to the known DNA-primase component p49. The recombinant GANP fragment covering this region synthesizes RNA primers for extension by DNA polymerase I with single-stranded DNA templates in vitro. GANP DNA-primase activity is controlled by phosphorylation at Ser(502) that is induced by CD40-mediated signaling in vitro and in the germinal center B cells stimulated with antigen in vivo. Overexpression of ganp cDNA in Daudi B cells caused the increased DNA synthesis more than the levels of the mock-transfectants. These evidences suggested that the novel DNA-primase GANP is involved in regulation of cell proliferation of antigen-driven B cells in germinal centers.
Figures
Figure 1
Intrinsic DNA-primase activity in GANP. (A) A putative GANP-PD. The GANP sequence (from amino acid 414 to 550) is compared with a DNA-primase p49 subunit (from amino acid 171 to 301). (B) In vitro DNA-primase assay. The GANP-PD was tagged with 6 × His and expressed in Escherichia coli to purify the recombinant GANP-PD. DNA-primase activity is measured by the incorporation of [3H]dTTP into M13 ssDNA with DNA polymerase. As a positive control, pol α that contains endogenous primase activity shows efficient DNA synthesis in the presence of four ribonucleotides to initiate RNA primers at first. As a negative control, the primase activity is measured with the recombinant GANP-PD in the absence of pol I. Activity of GANP-PD is measured in the presence of pol I with or without four ribonucleotides, or with only ATP. Effects of the RNA polymerase, including primase inhibitors, are examined to confirm the primase activity. (C Left) In vitro kinase assay with Cdk2. The recombinant GANP-PD was phosphorylated with cellular Cdk2 complex immunoprecipitated with anti-Cdk2 Ab. Incorporation of [γ-32P]ATP was visualized by autoradiography after SDS/PAGE. The 49-kDa GANP-PD and the degradation products are phosphorylated. (Right) Mutant GANP-PD was prepared by the substitution of Ser502 to Ala (502S/A) or Glu (502S/E). Primase activity was measured in the presence of pol I with the recombinant protein as in Fig. 1_B_. All data were confirmed by the repeated experiments.
Figure 2
Effect of over-expressed ganp in cell cycling. _Ganp_- and _mock-_transfectants were treated with the PI solution and characterized for cell size and DNA ploidity. Flow cytometric analysis was carried out with side scatter (SSC) and forward scatter (FSC) or with PI staining.
Figure 3
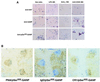
Induction of phosphorylated form of GANP DNA-primase in activated B cells in vitro and in vivo. (A) Normal spleen B cells were stimulated in vitro for 48 h with the stimulatory reagents as indicated. GANP expression was detected by anti-GANP mAb, and the induction of phosphorylation at Ser502 was detected by anti-pSer502 (PG/103) mAb in comparison with a control anti-glutathione _S_-transferase mAb. (B) The spleen sections from the TNP-KLH-immunized mice were stained histochemically as described in Materials and Methods. (Left) Most of pSer502 signal (blue) overlaps with PNA+ cells (brown). (Center) pSer502 signal (blue) appears up-regulated selectively in GC-B cells surrounded with IgD+ cells (brown). (Right) The up-regulation of pSer502 signal (blue) is detected in both CR1+ (brown) and CR1− area of GCs. The sections were prepared by the serial manner so that the profiles of the multiple markers are to be superimposed.
Similar articles
- Involvement of GANP in B cell activation in T cell-dependent antigen response.
Sakaguchi N, Fujimura S, Kuwahara K. Sakaguchi N, et al. Dev Immunol. 2002 Sep;9(3):169-72. doi: 10.1080/1044667031000137647. Dev Immunol. 2002. PMID: 12885157 Free PMC article. - A novel nuclear phosphoprotein, GANP, is up-regulated in centrocytes of the germinal center and associated with MCM3, a protein essential for DNA replication.
Kuwahara K, Yoshida M, Kondo E, Sakata A, Watanabe Y, Abe E, Kouno Y, Tomiyasu S, Fujimura S, Tokuhisa T, Kimura H, Ezaki T, Sakaguchi N. Kuwahara K, et al. Blood. 2000 Apr 1;95(7):2321-8. Blood. 2000. PMID: 10733502 - PU.1 is involved in the regulation of B lineage-associated and developmental stage-dependent expression of the germinal center-associated DNA primase GANP.
EL-Gazzar MA, Maeda K, Nomiyama H, Nakao M, Kuwahara K, Sakaguchi N. EL-Gazzar MA, et al. J Biol Chem. 2001 Dec 21;276(51):48000-8. doi: 10.1074/jbc.M106696200. Epub 2001 Oct 18. J Biol Chem. 2001. PMID: 11641399 - [Role of GANP in B cell activation].
Kuwahara K, Sakaguchi N. Kuwahara K, et al. Tanpakushitsu Kakusan Koso. 2002 Dec;47(16 Suppl):2300-5. Tanpakushitsu Kakusan Koso. 2002. PMID: 12518452 Review. Japanese. No abstract available. - Germinal Center B-Cell-Associated Nuclear Protein (GANP) Involved in RNA Metabolism for B Cell Maturation.
Sakaguchi N, Maeda K. Sakaguchi N, et al. Adv Immunol. 2016;131:135-86. doi: 10.1016/bs.ai.2016.02.003. Epub 2016 Mar 29. Adv Immunol. 2016. PMID: 27235683 Review.
Cited by
- The mRNA export machinery requires the novel Sac3p-Thp1p complex to dock at the nucleoplasmic entrance of the nuclear pores.
Fischer T, Strässer K, Rácz A, Rodriguez-Navarro S, Oppizzi M, Ihrig P, Lechner J, Hurt E. Fischer T, et al. EMBO J. 2002 Nov 1;21(21):5843-52. doi: 10.1093/emboj/cdf590. EMBO J. 2002. PMID: 12411502 Free PMC article. - Humanized Immunoglobulin Mice: Models for HIV Vaccine Testing and Studying the Broadly Neutralizing Antibody Problem.
Verkoczy L. Verkoczy L. Adv Immunol. 2017;134:235-352. doi: 10.1016/bs.ai.2017.01.004. Adv Immunol. 2017. PMID: 28413022 Free PMC article. Review. - GANP-mediated recruitment of activation-induced cytidine deaminase to cell nuclei and to immunoglobulin variable region DNA.
Maeda K, Singh SK, Eda K, Kitabatake M, Pham P, Goodman MF, Sakaguchi N. Maeda K, et al. J Biol Chem. 2010 Jul 30;285(31):23945-53. doi: 10.1074/jbc.M110.131441. Epub 2010 May 27. J Biol Chem. 2010. PMID: 20507984 Free PMC article. - Eukaryotic MCM proteins: beyond replication initiation.
Forsburg SL. Forsburg SL. Microbiol Mol Biol Rev. 2004 Mar;68(1):109-31. doi: 10.1128/MMBR.68.1.109-131.2004. Microbiol Mol Biol Rev. 2004. PMID: 15007098 Free PMC article. Review. - Protein phosphatase subunit G5PR is needed for inhibition of B cell receptor-induced apoptosis.
Xing Y, Igarashi H, Wang X, Sakaguchi N. Xing Y, et al. J Exp Med. 2005 Sep 5;202(5):707-19. doi: 10.1084/jem.20050637. Epub 2005 Aug 29. J Exp Med. 2005. PMID: 16129705 Free PMC article.
References
- MacLennan I C M. Annu Rev Immunol. 1994;12:117–139. - PubMed
- Kelsoe G. Immunity. 1996;4:107–111. - PubMed
- Rajewsky K. Nature (London) 1996;381:751–758. - PubMed
- Kosco-Vilbois M H, Zentgraf H, Gerdes J, Bonnefoy J-H. Immunol Today. 1997;18:225–230. - PubMed
- Kuwahara K, Yoshida M, Kondo E, Sakata A, Watanabe Y, Abe E, Kouno Y, Tomiyasu S, Fujimura S, Tokuhisa T, Kimura H, Ezaki T, Sakaguchi N. Blood. 2000;95:2321–2328. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials