Enhanced ROS production in oncogenically transformed cells potentiates c-Jun N-terminal kinase and p38 mitogen-activated protein kinase activation and sensitization to genotoxic stress - PubMed (original) (raw)
Enhanced ROS production in oncogenically transformed cells potentiates c-Jun N-terminal kinase and p38 mitogen-activated protein kinase activation and sensitization to genotoxic stress
M Benhar et al. Mol Cell Biol. 2001 Oct.
Abstract
Many primary tumors as well as transformed cell lines display high sensitivity to chemotherapeutic drugs and radiation. The molecular mechanisms that underlie this sensitivity are largely unknown. Here we show that the sensitization of transformed cells to stress stimuli is due to the potentiation of the c-Jun N-terminal kinase (JNK) and p38 mitogen-activated protein kinase pathways. Activation of these pathways by the antitumor drug cis-platin (CDDP) and by other stress agents is markedly enhanced and is induced by lower stress doses in NIH 3T3 cells overexpressing epidermal growth factor receptor, HER1-2 kinase, or oncogenic Ras than in nontransformed NIH 3T3 cells. Inhibition of stress kinase activity by specific inhibitors reduces CDDP-mediated cell death in transformed cells, whereas overactivation of stress kinase pathways augments cells death. Potentiation of stress kinases is a common feature of cells transformed by different oncogenes, including cells derived from human tumors, and is shown here to be independent of the activity of the particular transforming oncoprotein. We further show that the mechanism that underlies potentiation of stress kinases in transformed cells involves reactive oxygen species (ROS), whose production is elevated in these cells. JNK/p38 activation is inhibited by antioxidants and in particular by inhibitors of the mitochondrial respiratory chain and NADPH oxidase. Conversely, by artificially elevating ROS levels in nontransformed NIH 3T3 cells we were able to induce potentiation of JNK/p38 activation. Taken together, our findings suggest that ROS-dependent potentiation of stress kinase pathways accounts for the sensitization of transformed cells to stress and anticancer drugs.
Figures
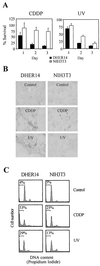
FIG. 1
Potentiation of cell death induced by CDDP and UV in NIH 3T3 cells that overexpress the EGFR. (A) Survival of DHER14 and NIH 3T3 cells following treatment with CDDP (30 μM) or UV irradiation (40 J/m2). The fraction of surviving cells was determined by the automated microculture methylene blue assay (as described in Materials and Methods). (B) TUNEL analysis of DHER14 and NIH 3T3 cells exposed to CDDP (30 μM) or UV irradiation (80 J/m2). Cell cultures were treated as indicated and subjected to TUNEL analysis 24 h after treatment. (C) FACS analysis of cells 48 h after CDDP (30 μM) or UV treatment (80 J/m2). The horizontal bar denotes the position of cells with sub-G1 DNA content, indicative of apoptosis. The percentage of such cells out of the total population is listed for each culture.
FIG. 2
Potentiation of JNK1 and p38 activation in DHER14 cells. DHER14 or NIH 3T3 cells were treated with CDDP for 16 h (A), UV radiation (analysis 1 h after irradiation) (B), or anisomycin for 1 h (C). JNK1 activity was measured by immunocomplex kinase assay, using GST–c-Jun as a substrate. The amount of JNK1 in cell lysates was measured by immunoblotting using anti-JNK1 antibodies. The activation of p38 was measured by immunoblotting, using antibodies that recognize the doubly phosphorylated (activated) form of p38. Identical blots run in parallel were reacted with anti-p38 antibodies. (D) Activation of ERK1/2. Cells were treated with CDDP for 16 h at the doses indicated. The activation of ERK1 and ERK2 was measured by immunoblotting, using antibodies that recognize the doubly phosphorylated (activated) forms of ERK1 and ERK2. Identical blots run in parallel were reacted with anti-ERK2 antibodies. Due to cross-reactivity the anti-ERK2 antibodies recognize both ERK isoforms: ERK2 (p42, lower band) and ERK1 (p44, upper band). (E) Activation of SEK1 and MKK3/6 was measured by immunoblotting, using antibodies that recognize the phosphorylated (activated) forms of SEK1 and MKK3/6.
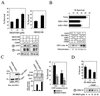
FIG. 3
JNK and p38 activation is involved in CDDP-induced cell death in DHER14 cells. (A) Cells were pretreated for 1 h with SB203580 at the doses indicated or with 20 μM SB202190 before addition of 30 μM CDDP. Survival was determined after 48 h by the automated microculture methylene blue assay (as described in Materials and Methods). In addition, the effect of SB203580 and SB202190 on ATF2 and c-Jun phosphorylation was measured (16 h after addition of the inhibitor) by immunoblotting, using antibodies which recognize the phosphorylated form of ATF2 and c-Jun. (B) Upper panel: DHER14 cells were cotransfected with GFP plasmid (1 μg) and empty vector or plasmids expressing kinases (2 μg each) as indicated. Twenty-four hours after transfection, cells were treated with 30 μM CDDP. Thirty hours after treatment, floating and attached cells were pooled and analyzed by FACS. Percent survival was defined as follows: (number of GFP-expressing cells in the CDDP-treated group/number of GFP-expressing cells in the nontreated group) × 100. Lower panel: DHER14 cells were transfected with empty vector, HA-JNK1, SEK1, and ASK1 as indicated. Twenty-four hours posttransfection, cells were treated with CDDP (100 μM) for 16 h. HA-JNK activity was determined by immunocomplex kinase assay. (C) Expression of GST-SEK1 in DHER14/SEK1 cells was detected by immunoblotting using anti-SEK1 antibodies. Stable transfectants of DHER14 cells expressing SEK1 (DHER14/SEK1) and DHER14 cells were exposed to 100 μM CDDP. JNK activation (16 h posttreatment) was measured by immunoblotting using anti-phospho-JNK antibodies. DHER14/SEK1 and DHER14 cells were exposed to CDDP (30 μM). Survival was measured as described above at the times indicated. (D) DHER14 cells were pretreated with PD98059 for 1 h before addition of 30 μM CDDP. Survival after 30 h was determined as above. Bottom panel: DHER14 cells were pretreated with PD98059 for 1 h before the addition of 100 μM CDDP. Cells were lysed 16 h after treatment, and ERK1 and ERK2 activation was determined as described for Fig. 2.
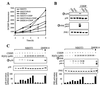
FIG. 4
Analysis of CDDP-induced cell death and JNK/p38 activation in CSH12 and NIH 3T3/Ras cells. (A) Survival of CSH12, NIH 3T3/Ras, and NIH 3T3 cells following treatment with CDDP (30 μM). Survival after 2 days was determined by the automated microculture methylene blue assay. (B and C) CSH12, NIH 3T3/Ras, or NIH 3T3 cells were treated with CDDP for 16 h at the indicated doses. The activation of JNK1 and p38 was determined as done for Fig. 2. Graphs show the data as quantified by densitometry of immunoblots using NIH image 1.61. Activation was calculated by dividing the densitometry values for the phosphoprotein by that of the total protein. (D) NIH 3T3/Ras or NIH 3T3 cells were treated with CDDP for 16 h at the indicated doses. The activation of ERK1/2 was determined as for Fig. 2.
FIG. 5
JNK and p38 activation is largely independent of the EGFR in DHER14 cells. (A) DHER14 cells were stimulated with 10 nM EGF for the indicated times. Activation of MAPKs was measured by immunoblotting. (B) DHER14 cells were pretreated with AG1478 (EGFR kinase inhibitor) at the indicated concentrations for 1 h before addition of EGF (10 nM) for 10 min. Cells were lysed and subjected to Western blot analysis using antiphosphotyrosine (PY) or anti-EGFR specific antibodies. (C) Cells were treated with CDDP (100 μM) for 16 h. During the last hour of incubation, AG1478 was added at the indicated concentrations. Cells were lysed and subjected to Western blot analysis, using anti-phospho-JNK, anti-phospho-p38, or anti-phospho-ERK1/2 specific antibodies. Parallel blots were reacted with anti-JNK1, anti-p38, or anti-ERK2 antibodies. Anti-phospho-JNK antibodies recognize the phosphorylated form of the p46 (JNK1) and p54 (JNK2) isoforms. Graphs show the percent activation as quantified by densitometry of immunoblots using NIH image 1.61. The phosphorylation observed in cells treated with CDDP in the absence of AG1478 was defined as 100%. Results are representative of three independent experiments. (D) Upper panel: DHER14 cells were pretreated with 1 μg of EGF per ml for 1 h. The EGF-containing medium was then removed, and cells were kept in regular medium for the times indicated. EGFR expression was measured by immunoblotting. Bottom panels: DHER14 cells were pretreated with 1 μg of EGF per ml for 1 h. The EGF-containing medium was then removed, and cells were kept in regular medium for 12 h prior to treatment with CDDP (100 μM) for an additional 12 h. Cells were lysed, and EGFR expression or MAPK activation was analyzed by immunoblotting. Graphs show the data as quantified by densitometry of immunoblots using NIH image 1.61.
FIG. 6
Involvement of ROS in potentiation of JNK/p38 activation in transformed NIH 3T3 cells. (A) Kinetics of ROS production in nonstimulated and CDDP-treated cells. Cells were labeled with the fluorescent dye DCDHF, treated as indicated, and analyzed as described in Material and Methods. Points on the graph are the average values from duplicate samples. Data are from a representative experiment, which was repeated three times with comparable results. (B) DHER14 cells were treated with 5 mM reduced GSH or NAC for 1 h, followed by treatment with 100 μM CDDP. After 16 h lysates were prepared and assayed for JNK or p38 activation by Western blot analysis as done for Fig. 5. (C) NIH 3T3 cells were treated once with H2O2 and/or CDDP at the doses indicated. After 16 h cells were lysed, and JNK or p38 activation was determined by immunoblotting.
FIG. 7
Implication of mitochondria and NADPH oxidase in CDDP-induced ROS generation in DHER14 cells. (A) DHER14 cells were incubated with CDDP (100 μM) for 16 h in the absence or presence of rotenone (Rot., 100 μM), DPI (10 μM), or NAC (1 mM). Cell lysates were assayed for JNK or p38 activation by Western blot analysis. (B) DHER14 cells were labeled with the fluorescent dye DCDHF and were treated with CDDP (100 μM) together with rotenone (Rot., 100 μM), DPI (10 μM), or NAC (1 mM) as indicated. The production of ROS was monitored as described in Materials and Methods. (C) Survival of DHER14 cells following CDDP treatment (30 μM) in the presence of rotenone (Rot., 100 μM) or DPI (10 μM). The fraction of surviving cells was determined by the automated microculture methylene blue assay (as described in Materials and Methods). (D) DHER14 cells were treated for 1 h with vehicle (no inhibitor), 300 nM AG1478, or 1 μM AG1478. ROS production was measured during the following 8 h in the absence of CDDP (left panel) or in the presence of 100 μM CDDP (right panel).
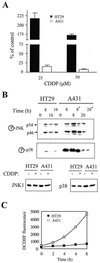
FIG. 8
Analysis of CDDP-induced cell death, JNK/p38 activation, and ROS production in human tumor cells. (A) Survival of A431 and HT29 cells 72 h following treatment with CDDP. The fraction of surviving cells was determined by the automated microculture methylene blue assay (as described in Materials and Methods). (B) A431 or HT29 cells were treated with 100 μM CDDP. The activation of JNK1 and p38 was measured by immunoblotting, using anti-phospho-JNK or anti-phospho-p38 specific antibodies. For comparison, levels of JNK and p38 in HT29 and A431 cells before or 8 h after CDDP treatment are shown. Two 16-h samples of HT29 cells and two 8-h samples of A431 cells were included in the analysis. Due to massive apoptosis in A431 cells, floating cells were collected and treated as a separate sample (marked by an asterisk). (C) Kinetics of ROS production in A431 and HT29 cells. Cells were labeled with the fluorescent dye DCDHF and were analyzed for ROS generation as described in Materials and Methods.
Similar articles
- c-Myc potentiates the mitochondrial pathway of apoptosis by acting upstream of apoptosis signal-regulating kinase 1 (Ask1) in the p38 signalling cascade.
Desbiens KM, Deschesnes RG, Labrie MM, Desfossés Y, Lambert H, Landry J, Bellmann K. Desbiens KM, et al. Biochem J. 2003 Jun 1;372(Pt 2):631-41. doi: 10.1042/BJ20021565. Biochem J. 2003. PMID: 12646044 Free PMC article. - Roles of JNK, p38 and ERK mitogen-activated protein kinases in the growth inhibition and apoptosis induced by cadmium.
Chuang SM, Wang IC, Yang JL. Chuang SM, et al. Carcinogenesis. 2000 Jul;21(7):1423-32. Carcinogenesis. 2000. PMID: 10874022 - Signalling for survival and death in neurones: the role of stress-activated kinases, JNK and p38.
Harper SJ, LoGrasso P. Harper SJ, et al. Cell Signal. 2001 May;13(5):299-310. doi: 10.1016/s0898-6568(01)00148-6. Cell Signal. 2001. PMID: 11369511 Review. - Novel components of mammalian stress-activated protein kinase cascades.
Kiefer F, Tibbles LA, Lassam N, Zanke B, Iscove N, Woodgett JR. Kiefer F, et al. Biochem Soc Trans. 1997 May;25(2):491-8. doi: 10.1042/bst0250491. Biochem Soc Trans. 1997. PMID: 9191142 Review. No abstract available.
Cited by
- Role of p38 and JNK MAPK signaling pathways and tumor suppressor p53 on induction of apoptosis in response to Ad-eIF5A1 in A549 lung cancer cells.
Taylor CA, Zheng Q, Liu Z, Thompson JE. Taylor CA, et al. Mol Cancer. 2013 May 2;12:35. doi: 10.1186/1476-4598-12-35. Mol Cancer. 2013. PMID: 23638878 Free PMC article. - Systems biology modeling reveals a possible mechanism of the tumor cell death upon oncogene inactivation in EGFR addicted cancers.
Zhou JP, Chen X, Feng S, Luo SD, Pan YL, Zhong L, Ji P, Wang ZR, Ma S, Li LL, Wei YQ, Yang SY. Zhou JP, et al. PLoS One. 2011;6(12):e28930. doi: 10.1371/journal.pone.0028930. Epub 2011 Dec 14. PLoS One. 2011. PMID: 22194952 Free PMC article. - Chronic low vitamin intake potentiates cisplatin-induced intestinal epithelial cell apoptosis in WNIN rats.
Vijayalakshmi B, Sesikeran B, Udaykumar P, Kalyanasundaram S, Raghunath M. Vijayalakshmi B, et al. World J Gastroenterol. 2006 Feb 21;12(7):1078-85. doi: 10.3748/wjg.v12.i7.1078. World J Gastroenterol. 2006. PMID: 16534849 Free PMC article. - Autophagy inhibition enhances therapy-induced apoptosis in a Myc-induced model of lymphoma.
Amaravadi RK, Yu D, Lum JJ, Bui T, Christophorou MA, Evan GI, Thomas-Tikhonenko A, Thompson CB. Amaravadi RK, et al. J Clin Invest. 2007 Feb;117(2):326-36. doi: 10.1172/JCI28833. Epub 2007 Jan 18. J Clin Invest. 2007. PMID: 17235397 Free PMC article. - H(2)O (2)-induced secretion of tumor necrosis factor-α evokes apoptosis of cardiac myocytes through reactive oxygen species-dependent activation of p38 MAPK.
Chen Z, Jiang H, Wan Y, Bi C, Yuan Y. Chen Z, et al. Cytotechnology. 2012 Jan;64(1):65-73. doi: 10.1007/s10616-011-9392-3. Epub 2011 Oct 15. Cytotechnology. 2012. PMID: 22002864 Free PMC article.
References
- Adler V, Yin Z, Tew K D, Ronai Z. Role of redox potential and reactive oxygen species in stress signaling. Oncogene. 1999;18:6104–6111. - PubMed
- Alessi D R, Cuenda A, Cohen P, Dudley D T, Saltiel A R. PD 098059 is a specific inhibitor of the activation of mitogen-activated protein kinase kinase in vitro and in vivo. J Biol Chem. 1995;270:27489–27494. - PubMed
- Arteaga C L, Winnier A R, Poirier M C, Lopez-Larraza D M, Shawver L K, Hurd S D, Stewart S J. p185c-erbB-2 signal enhances cisplatin-induced cytotoxicity in human breast carcinoma cells: association between an oncogenic receptor tyrosine kinase and drug-induced DNA repair. Cancer Res. 1994;54:3758–3765. - PubMed
- Basu A, Cline J S. Oncogenic transformation alters cisplatin-induced apoptosis in rat embryo fibroblasts. Int J Cancer. 1995;63:597–603. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous