Prolonged morphine treatment targets delta opioid receptors to neuronal plasma membranes and enhances delta-mediated antinociception - PubMed (original) (raw)
Prolonged morphine treatment targets delta opioid receptors to neuronal plasma membranes and enhances delta-mediated antinociception
C M Cahill et al. J Neurosci. 2001.
Abstract
Opioid receptors are known to undergo complex regulatory changes in response to ligand exposure. In the present study, we examined the effect of morphine on the in vitro and in vivo density and trafficking of delta opioid receptors (deltaORs). Prolonged exposure (48 hr) of cortical neurons in culture to morphine (10 microm) resulted in a robust increase in the internalization of Fluo-deltorphin, a highly selective fluorescent deltaOR agonist. This effect was mu-mediated because it was entirely blocked by the selective mu opioid receptor antagonist d-Phe-Cys-Tyr-d-Trp-Orn-Thr-Pen-Thr-NH(2) and was reproduced using the selective mu agonist fentanyl citrate. Immunogold electron microscopy revealed a marked increase in the cell surface density of deltaORs in neurons exposed to morphine, indicating that the increase in Fluo-deltorphin internalization was caused by increased receptor availability. Prolonged morphine exposure had no effect on deltaOR protein levels, as assessed by immunocytochemistry and Western blotting, suggesting that the increase in bioavailable deltaORs was caused by recruitment of reserve receptors from intracellular stores and not from receptor neosynthesis. Complementary in vivo studies demonstrated that chronic treatment of adult rats with morphine (5-15 mg/kg, s.c., every 12 hr) similarly augmented targeting of deltaORs to neuronal plasma membranes in the dorsal horn of the spinal cord. Furthermore, this treatment markedly potentiated intrathecal d-[Ala(2)]deltorphin II-induced antinociception. Taken together, these results demonstrate that prolonged stimulation of neurons with morphine markedly increases recruitment of intracellular deltaORs to the cell surface, both in vitro and in vivo. We propose that this type of receptor subtype cross-mobilization may widen the transduction repertoire of G-protein-coupled receptors and offer new therapeutic strategies.
Figures
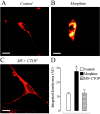
Fig. 1.
Chronic stimulation with morphine leads to μOR-induced increase in bioavailable δORs at the plasma membrane. Internalization of Fluo-DLT, a selective δOR agonist, in primary cortical neurons either untreated (A,Control) or treated with 10 μ
m
morphine sulfate for 48 hr (B) or treated with 10 μ
m
morphine sulfate (MS) in the presence of 10 μ
m
of the μOR antagonist CTOP (C). Images are displayed in pseudocolor, where_white_ represents the highest fluorescence intensity and_red_ represents the lowest. Internalized Fluo-DLT can clearly be seen intracellularly, especially in morphine-treated cells. Note the absence of internalized ligand in the nucleus. Scale bar, 10 μm. D, Internalization of Fluo-DLT is significantly increased (p < 0.001) after treatment with morphine for 48 hr. This augmentation is no longer observed when morphine is administered in the presence of CTOP. Each_bar_ in the graph represents the integrated density per area (±SEM) pooled from at least three different experiments, with_n_ = 13–38 for each group. Statistical significance was determined using the Kruskal–Wallis test, followed by Dunn's multiple comparison test. The asterisk denotes significant differences between morphine-treated and untreated neurons (p < 0.001), as well as between morphine- and _MS+CTOP_-treated neurons (p < 0.001).
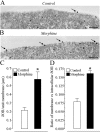
Fig. 2.
Morphine leads to increased plasma membrane-associated δORs as assessed by immunogold electron microscopy. Primary cortical cells were maintained in culture for 6–8 d before treatment, or not, with 10 μ
m
morphine sulfate for 48 hr, and subsequent processing for immunogold staining of δORs. In untreated (A, Control) cortical neurons, immunogold particles are rarely associated with the cell surface (arrow), whereas in morphine-treated neurons (B), cell surface-associated immunogold particles are more numerous (arrows). Scale bar, 0.5 μm.C, The number of membrane-associated gold particles per length of plasma membrane is significantly increased in primary cortical neurons treated for 48 hr with 10 μ
m
morphine when compared with untreated controls. The asterisk_indicates significant difference (two-tailed Mann–Whitney_U test; p < 0.002) between morphine-treated and untreated neurons. D, The proportion of membrane-associated gold particles (expressed as the ratio of membrane-associated versus intracellular δORs per neuron) is significantly increased in primary cortical neurons treated for 48 hr with 10 μ
m
morphine when compared with untreated controls, indicating a shift in receptors from an intracellular location toward the cell surface. The asterisk in_D_ indicates significant difference (two-tailed Mann–Whitney U test, p < 0.0001) between morphine-treated and untreated neurons.
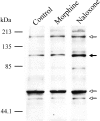
Fig. 3.
Comparison of the effect of naloxone and morphine on total δOR protein expression. Dissociated primary cortical neurons were maintained in culture for 6–8 d and treated, or not, with 10 μ
m
naloxone or 10 μ
m
morphine sulfate for 48 hr. Cell membranes were isolated, and the samples were resolved and immunoblotted with the δOR antisera. Major immunoreactive bands were observed at estimated molecular weights of 52, 59, 105, and 180 kDa (arrows). Specificity of this antibody has been characterized previously (Cahill et al., 2001). Immunoblot analysis reveals that treatment of cortical cells with 10 μ
m
naloxone for 48 hr, but not 10 μ
m
morphine for 48 hr, leads to an increased signal intensity of the band at 105 kDa (filled arrow), indicating augmented δOR protein expression. This increase was reproduced in three experiments.
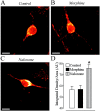
Fig. 4.
Immunocytochemical determination of the effects of morphine and naloxone on δOR protein expression. Treatment with 10 μ
m
naloxone (C) for 48 hr leads to a significant increase in fluorescent immunolabeling intensity when compared with either untreated (A) or morphine-treated neurons (B). Dissociated primary cortical cells were maintained in culture for 6–8 d, treated or not with 10 μ
m
naloxone or 10 μ
m
morphine sulfate for 48 hr, and stained with the anti-δOR antibody and a Cy3-linked secondary antibody. Control refers to untreated neurons. Fluorescent images were acquired using a confocal microscope as described in Figure 1. Scale bar, 10 μm.D, As detected by immunocytochemistry, treatment with naloxone results in a significant increase in δOR protein levels when compared with either morphine-treated or untreated neurons. Each_bar_ in the graph represents the integrated density per area (±SEM) pooled from three different experiments, with_n_ = 8 for each condition in each experiment. A two-tailed Mann–Whitney U test with a Bonferroni correction was used to determine statistical significance. The_asterisk_ denotes significant differences between naloxone-treated and untreated neurons (U statistic = 106; p < 0.0005), as well as between morphine- and naloxone-treated neurons (U statistic = 107;p < 0.0005). There was no significant difference between untreated and morphine-treated neurons.
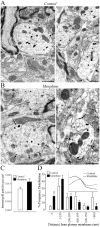
Fig. 5.
Electron micrographs of δOR-immunolabeled dendrites in the superficial dorsal horn of the spinal cord in saline- and chronic morphine-treated rats (n = 3–4 per group). In saline-treated rats (A), few immunogold particles are evident on the plasma membrane (arrows), whereas in morphine-treated rats (B), several immunogold particles are associated with it (arrows). Ultrastructural analysis reveals no significant difference in the number of gold particles per unit area of labeled dendritic profiles between treatment groups (C). However, the percentage of gold particles associated with the plasma membrane is significantly higher in rats injected with morphine compared with saline-treated rats (D, first column). A shortening of the mean distance separating intracellular immunogold particles from the plasma membrane is also observed (D, inset; p < 0.0001). Statistical analysis comparing the pattern of labeling was performed using the Mann–Whitney U test (two tailed) on the percentage of receptors localized in each divided compartment (distance for the plasma membrane) as well as the mean distance from the plasma membrane. Scale bar, 0.5 μm.
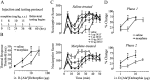
Fig. 6.
Rats chronically treated with morphine exhibit enhanced antinociceptive effects of intrathecal δOR agonist in both thermal acute pain response (B) and tonic pain (C, D) compared with control rats.A, Diagram illustrating the testing and treatment regimen for morphine and saline injections of rats. The dose administered is indicated by arrows at each specified time point (hours). B, Response threshold to intrathecal administration of
d
-[Ala2]Deltorphin II (DELT) in the hot plate test (n = 6 per group). Statistical analysis using a two-tailed unpaired t test revealed a significant difference between groups (p < 0.05), as denoted by the asterisk. C, Nocifensive behaviors assessed using a weighed score produced by intraplantar injection of formalin (n = 5–6 per group). All testing was performed 8–20 hr after the final injection of morphine.D, Area under the curve values for phase 1 (time 0–10 min) and phase 2 (time 15–40 min) of the formalin test were converted to percentage change from control in respective groups to obtain antinociceptive dose–response curves. ED50 values for the first phase of the formalin test are 3.19 μg for morphine-treated and 7.71 μg for control rats. ED50 values for the second phase of the formalin response are 4.9 and 32.4 μg for morphine-treated and control rats, respectively.
Fig. 7.
Schematic representation of the ligand-induced receptor internalization assay used to assess the density of plasma membrane-associated δOR receptors. At low receptor density on the plasma membrane, a small number of receptors can bind and internalize their cognate ligand at the permissive temperature of 37°C. If, on the other hand, the receptor density on the plasma membrane is high at the start of the internalization assay, a greater number of receptors can undergo ligand-induced receptor internalization. Surface-bound ligand is removed by hypertonic acid-wash after ligand incubation to ensure that only intracellular ligand is detected. Chronic stimulation with morphine leads to μOR-induced increase in bioavailable δORs at the plasma membrane.
Similar articles
- Regulation of delta-opioid receptor trafficking via mu-opioid receptor stimulation: evidence from mu-opioid receptor knock-out mice.
Morinville A, Cahill CM, Esdaile MJ, Aibak H, Collier B, Kieffer BL, Beaudet A. Morinville A, et al. J Neurosci. 2003 Jun 15;23(12):4888-98. doi: 10.1523/JNEUROSCI.23-12-04888.2003. J Neurosci. 2003. PMID: 12832511 Free PMC article. - Morphine-induced in vivo release of spinal cholecystokinin is mediated by delta-opioid receptors--effect of peripheral axotomy.
Gustafsson H, Afrah AW, Stiller CO. Gustafsson H, et al. J Neurochem. 2001 Jul;78(1):55-63. doi: 10.1046/j.1471-4159.2001.00393.x. J Neurochem. 2001. PMID: 11432973 - Morphine-induced changes in delta opioid receptor trafficking are linked to somatosensory processing in the rat spinal cord.
Morinville A, Cahill CM, Aibak H, Rymar VV, Pradhan A, Hoffert C, Mennicken F, Stroh T, Sadikot AF, O'Donnell D, Clarke PB, Collier B, Henry JL, Vincent JP, Beaudet A. Morinville A, et al. J Neurosci. 2004 Jun 16;24(24):5549-59. doi: 10.1523/JNEUROSCI.2719-03.2004. J Neurosci. 2004. PMID: 15201327 Free PMC article. - Opioid receptor heteromers in analgesia.
Costantino CM, Gomes I, Stockton SD, Lim MP, Devi LA. Costantino CM, et al. Expert Rev Mol Med. 2012 Apr 10;14:e9. doi: 10.1017/erm.2012.5. Expert Rev Mol Med. 2012. PMID: 22490239 Free PMC article. Review. - Functional relevance of μ-δ opioid receptor heteromerization: a role in novel signaling and implications for the treatment of addiction disorders: from a symposium on new concepts in mu-opioid pharmacology.
Stockton SD Jr, Devi LA. Stockton SD Jr, et al. Drug Alcohol Depend. 2012 Mar 1;121(3):167-72. doi: 10.1016/j.drugalcdep.2011.10.025. Epub 2011 Nov 23. Drug Alcohol Depend. 2012. PMID: 22115888 Free PMC article. Review.
Cited by
- Opioid modulation of prefrontal cortex cells and circuits.
Cole RH, Moussawi K, Joffe ME. Cole RH, et al. Neuropharmacology. 2024 May 1;248:109891. doi: 10.1016/j.neuropharm.2024.109891. Epub 2024 Feb 27. Neuropharmacology. 2024. PMID: 38417545 Review. - G-protein coupled receptor resensitization-appreciating the balancing act of receptor function.
Mohan ML, Vasudevan NT, Gupta MK, Martelli EE, Naga Prasad SV. Mohan ML, et al. Curr Mol Pharmacol. 2012 May 30. Online ahead of print. Curr Mol Pharmacol. 2012. PMID: 22697395 Free PMC article. - Essential role of mu opioid receptor in the regulation of delta opioid receptor-mediated antihyperalgesia.
Gendron L, Pintar JE, Chavkin C. Gendron L, et al. Neuroscience. 2007 Dec 19;150(4):807-17. doi: 10.1016/j.neuroscience.2007.09.060. Epub 2007 Oct 5. Neuroscience. 2007. PMID: 17997230 Free PMC article. - Regulation of delta-opioid receptor trafficking via mu-opioid receptor stimulation: evidence from mu-opioid receptor knock-out mice.
Morinville A, Cahill CM, Esdaile MJ, Aibak H, Collier B, Kieffer BL, Beaudet A. Morinville A, et al. J Neurosci. 2003 Jun 15;23(12):4888-98. doi: 10.1523/JNEUROSCI.23-12-04888.2003. J Neurosci. 2003. PMID: 12832511 Free PMC article. - Functional Divergence of Delta and Mu Opioid Receptor Organization in CNS Pain Circuits.
Wang D, Tawfik VL, Corder G, Low SA, François A, Basbaum AI, Scherrer G. Wang D, et al. Neuron. 2018 Apr 4;98(1):90-108.e5. doi: 10.1016/j.neuron.2018.03.002. Epub 2018 Mar 22. Neuron. 2018. PMID: 29576387 Free PMC article.
References
- Belcheva MM, Barg J, McHale R, Coscia CJ. Naltrexone induces down- and upregulation of delta opioid receptors in rat brain regions. Brain Res Bull. 1994;35:69–72. - PubMed
- Bilsky EJ, Bernstein RN, Hruby VJ, Rothman RB, Lai J, Porecca F. Characterization of antinociception to opioid receptor selective agonists after antisense oligodeoxynucleotide-mediated “knock-down” of opioid receptor in vivo. J Pharmacol Exp Ther. 1996;277:491–501. - PubMed
- Cahill CM, Lee M-C, Vincent JP, Beaudet A. Increase in delta opioid receptor internalization following chronic morphine treatment. Soc Neurosci Abstr. 1999;25:883.6.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials