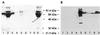Cell surface heparan sulfate is a receptor for human herpesvirus 8 and interacts with envelope glycoprotein K8.1 - PubMed (original) (raw)
Cell surface heparan sulfate is a receptor for human herpesvirus 8 and interacts with envelope glycoprotein K8.1
A Birkmann et al. J Virol. 2001 Dec.
Abstract
An immunodominant envelope glycoprotein is encoded by the human herpesvirus 8 (HHV-8) (also termed Kaposi's sarcoma-associated herpesvirus) K8.1 gene. The functional role of glycoprotein K8.1 is unknown, and recognizable sequence homology to K8.1 is not detectable in the genomes of most other closely related gammaherpesviruses, such as herpesvirus saimiri or Epstein-Barr virus. In search for a possible function for K8.1, we expressed the ectodomain of K8.1 fused to the Fc part of human immunoglobulin G1 (K8.1DeltaTMFc). K8.1DeltaTMFc specifically bound to the surface of cells expressing glycosaminoglycans but not to mutant cell lines negative for the expression of heparan sulfate proteoglycans. Binding of K8.1DeltaTMFc to mammalian cells could be blocked by heparin. Interestingly, the infection of primary human endothelial cells by HHV-8 could also be blocked by similar concentrations of heparin. The specificity and affinity of these interactions were then determined by surface plasmon resonance measurements using immobilized heparin and soluble K8.1. This revealed that K8.1 binds to heparin with an affinity comparable to that of glycoproteins B and C of herpes simplex virus, which are known to be involved in target cell recognition by binding to cell surface proteoglycans, especially heparan sulfate. We conclude that cell surface glycosaminoglycans play a crucial role in HHV-8 target cell recognition and that HHV-8 envelope protein K8.1 is at least one of the proteins involved.
Figures
FIG. 1
Purification of recombinant K8.1ΔTMFc fusion protein. Soluble K8.1 was expressed in a C-terminal fusion to the Fc part of human IgG1 containing a C-terminal Myc epitope (K8.1ΔTMFc). Both K8.1ΔTMFc and Fc alone (data not shown) were expressed in transiently transfected 293T cells and purified from the cell culture supernatant by affinity chromatography on protein A-Sepharose columns. An SDS-polyacrylamide gel stained with Coomassie brilliant blue (A) and a Western blot using a monoclonal antibody against K8.1 (BS555) (25) and a horseradish peroxidase-labeled secondary antibody against murine IgG (B) are shown. Lanes: 1, supernatant from 293T cells transfected with a negative control plasmid; 2, flowthrough supernatant after adsorption; 3 and 4, washing steps; 5 to 8, elution of K8.1ΔTMFc; 9, supernatant from 293T cells transfected with K8.1ΔTMFc expression plasmid prior to absorption.
FIG. 2
Binding of K8.1 on the surface of HMVEC-d. HMVEC-d were fixed with paraformaldehyde and preincubated with Cohn fraction II to avoid binding of K8.1ΔTMFc to cellular Fc receptors. Cells were then incubated with either K8.1ΔTMFc (A) or Fc alone (B), followed by incubation with a monoclonal antibody directed to the C-terminal Myc epitope. Using an anti-mouse IgG antibody labeled with the fluorescent dye Cy3, binding of K8.1ΔTMFc but not of Fc alone could then be detected.
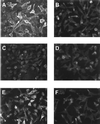
FIG. 3
K8.1ΔTMFc binds on the surface of mouse L cells (A) but not on the gro2C mutant (C) lacking heparan sulfate. Cells were fixed with paraformaldehyde and treated with Cohn fraction II derived from human plasma. Cells were then incubated with K8.1ΔTMFc (A, C, and E) or Fc alone (B, D, and F). Binding was detected with a mouse monoclonal antibody against the Myc epitope which was present at the C termini of both K8.1ΔTMFc and Fc, followed by incubation with a Cy3 labeled anti-mouse IgG antibody. Specific binding of K8.1ΔTMFc (A), but not of Fc alone (B), could be detected on mouse L cells. In contrast, K8.1ΔTMFc could not bind to the gro2C mutant of mouse L cells (22) lacking heparan sulfate expression (C and D). However, when cell line gro2C-EXT1, which is a derivative of gro2C with partially restored expression of heparan sulfate (28), was used, specific binding of K8.1ΔTMFc could again be observed (E and F).
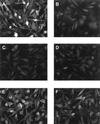
FIG. 4
Binding of K8.1ΔTMFc to mouse L cells is blocked by heparin. Mouse L cells were fixed with paraformaldehyde and treated with Cohn fraction II from human blood. Prior to incubation with the cells, K8.1ΔTMFc (A, C, D, E, and F) or soluble Fc (B) was preincubated with either PBS (A and B), 0.1 mg of heparin per ml (C), 1.0 mg of heparin per ml (D), 0.1 mg of chondroitin sulfate per ml (E), or 1.0 mg of chondroitin sulfate per ml (F). Whereas heparin at both 0.1 and 1.0 mg/ml efficiently blocks binding of K8.1ΔTMFc to mouse L cells (C and D), binding is still possible in the presence of chondroitin sulfate A at up to 1.0 mg/ml (E and F).
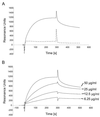
FIG. 5
(A) Binding of soluble K8.1 to a heparin-coated biosensor measured by SPR. The bindings of K8.1ΔTMFc (solid line) and Fc alone (dotted line) to a heparin biosensor are compared. Binding of K8.1 and Fc is shown as RU versus time in seconds. Both proteins were used at 50 μg/ml and injected at 4 μl/min onto the heparin-coated surface. The protein solution was injected for 300 s, followed by injection of running buffer at 4 μl/min for 250 s. The peak visible at 300 s is due to the change of the refractory index caused by replacing the buffer on the sensor chip. Due to the purification process, the buffer used to apply K8.1ΔTMFc differed slightly from the running buffer. (B) Binding of soluble K8.1 at concentrations of 6.25 to 50 μg/ml to a heparin-coated biosensor chip. K8.1ΔTMFc was injected at 4 μl/min for 300 s and reached a new equilibrium value with each higher concentration. Data from multiple runs without baseline subtraction are given as RU versus time in seconds. The peak at 300 s is due to the change of the refractory index when the protein solution was replaced with running buffer. The kinetic data shown in this figure were used to calculate the dissociation constants shown in Table 1.
FIG. 6
Competitive inhibition of K8.1ΔTMFc binding to a heparin-coated biosensor surface by heparin (A) or chondroitin sulfate A (CsA) (B). Soluble heparin at concentrations of 0 to 1.0 mg/ml (A) or soluble chondroitin sulfate A at concentrations of 0 to 10.0 mg/ml (B) was mixed with soluble K8.1 before application to the heparin-coated biosensor chip. Whereas heparin efficiently blocked K8.1 binding at concentrations as low as 0.1 mg/ml, hardly any effect was seen with chondroitin sulfate A even at the highest concentration.
FIG. 7
HHV-8 infection of human endothelial cells is blocked by heparin. Concentrated supernatant (200-fold) from BCBL-1 cells stimulated with TPA was used to infect HMVEC-d. Prior to application on the HMVEC-d, the concentrated supernatant was mixed with PBS (B), 0.2 mg of heparin per ml (C), 2.0 mg of heparin per ml (D), 0.2 mg of chondroitin sulfate A per ml (E), or 2.0 mg of chondroitin sulfate A per ml (F). Uninfected cells are shown in panel A. Cells were fixed with paraformaldehyde at 2 days postinfection, and IFA was performed using a mouse monoclonal antibody against K8.1 and a Cy3-labeled secondary antibody against mouse immunoglobulin. Immunofluorescence pictures are shown on the left, and phase-contrast pictures of the same cells are shown on the right. Expression of the lytic K8.1 protein and a typical cytopathogenic effect were seen when the inoculum was treated with PBS (B) or chondroitin sulfate (E and F) prior to infection. However, when preincubation was done with heparin at either 0.2 mg/ml (C) or 2.0 mg/ml (D), the number and size of the plaques were clearly reduced.
Similar articles
- Human herpesvirus 8 envelope-associated glycoprotein B interacts with heparan sulfate-like moieties.
Akula SM, Pramod NP, Wang FZ, Chandran B. Akula SM, et al. Virology. 2001 Jun 5;284(2):235-49. doi: 10.1006/viro.2001.0921. Virology. 2001. PMID: 11384223 - Human herpesvirus 8 envelope glycoprotein K8.1A interaction with the target cells involves heparan sulfate.
Wang FZ, Akula SM, Pramod NP, Zeng L, Chandran B. Wang FZ, et al. J Virol. 2001 Aug;75(16):7517-27. doi: 10.1128/JVI.75.16.7517-7527.2001. J Virol. 2001. PMID: 11462024 Free PMC article. - Role of the extracellular domain of human herpesvirus 7 glycoprotein B in virus binding to cell surface heparan sulfate proteoglycans.
Secchiero P, Sun D, De Vico AL, Crowley RW, Reitz MS Jr, Zauli G, Lusso P, Gallo RC. Secchiero P, et al. J Virol. 1997 Jun;71(6):4571-80. doi: 10.1128/JVI.71.6.4571-4580.1997. J Virol. 1997. PMID: 9151851 Free PMC article. - Heparan sulfate glycosaminoglycans as primary cell surface receptors for herpes simplex virus.
Spear PG, Shieh MT, Herold BC, WuDunn D, Koshy TI. Spear PG, et al. Adv Exp Med Biol. 1992;313:341-53. doi: 10.1007/978-1-4899-2444-5_33. Adv Exp Med Biol. 1992. PMID: 1332443 Review. - Herpes simplex virus: receptors and ligands for cell entry.
Spear PG. Spear PG. Cell Microbiol. 2004 May;6(5):401-10. doi: 10.1111/j.1462-5822.2004.00389.x. Cell Microbiol. 2004. PMID: 15056211 Review.
Cited by
- Kaposi's sarcoma-associated herpesvirus viral interferon regulatory factor 3 inhibits gamma interferon and major histocompatibility complex class II expression.
Schmidt K, Wies E, Neipel F. Schmidt K, et al. J Virol. 2011 May;85(9):4530-7. doi: 10.1128/JVI.02123-10. Epub 2011 Feb 23. J Virol. 2011. PMID: 21345951 Free PMC article. - Kaposi Sarcoma-Associated Herpesvirus Glycoprotein H Is Indispensable for Infection of Epithelial, Endothelial, and Fibroblast Cell Types.
Muniraju M, Mutsvunguma LZ, Foley J, Escalante GM, Rodriguez E, Nabiee R, Totonchy J, Mulama DH, Nyagol J, Wussow F, Barasa AK, Brehm M, Ogembo JG. Muniraju M, et al. J Virol. 2019 Jul 30;93(16):e00630-19. doi: 10.1128/JVI.00630-19. Print 2019 Aug 15. J Virol. 2019. PMID: 31142670 Free PMC article. - Kaposi's sarcoma-associated herpesvirus gH/gL: glycoprotein export and interaction with cellular receptors.
Hahn A, Birkmann A, Wies E, Dorer D, Mahr K, Stürzl M, Titgemeyer F, Neipel F. Hahn A, et al. J Virol. 2009 Jan;83(1):396-407. doi: 10.1128/JVI.01170-08. Epub 2008 Oct 22. J Virol. 2009. PMID: 18945775 Free PMC article. - A comparative review of viral entry and attachment during large and giant dsDNA virus infections.
Sobhy H. Sobhy H. Arch Virol. 2017 Dec;162(12):3567-3585. doi: 10.1007/s00705-017-3497-8. Epub 2017 Sep 2. Arch Virol. 2017. PMID: 28866775 Free PMC article. Review. - Kaposi sarcoma-associated herpesvirus complement control protein (KCP) and glycoprotein K8.1 are not required for viral infection in vitro or in vivo.
Muniraju M, Mutsvunguma LZ, Reidel IG, Escalante GM, Cua S, Musonda W, Calero-Landa J, Farelo MA, Rodriguez E, Li Z, Ogembo JG. Muniraju M, et al. J Virol. 2024 Jun 13;98(6):e0057624. doi: 10.1128/jvi.00576-24. Epub 2024 May 20. J Virol. 2024. PMID: 38767375 Free PMC article.
References
- Akula S M, Pramod N P, Wang F Z, Chandran B. Human herpesvirus 8 envelope-associated glycoprotein B interacts with heparan sulfate-like moieties. Virology. 2001;284:235–249. - PubMed
- Albini A, Aluigi M G, Benelli R, Berti E, Biberfeld P, Blasig C, Calabro M L, Calvo F, Chieco-Bianchi L, Corbellino M, Del Mistro A, Ekman M, Favero A, Hofschneider P H, Kaaya E, Lebbe C, Morel P, Neipel F, Noonan D M, Parravicini C, Repetto L, Schalling M, Stürzl M, Tschachler E. Oncogenesis in HIV-infection: KSHV and Kaposi's sarcoma. Int J Oncol. 1996;9:5–8. - PubMed
- Ambroziak J A, Blackbourn D J, Herndier B G, Glogau R G, Gullett J H, McDonald A R, Lennette E T, Levy J A. Herpes-like sequences in HIV-infected and uninfected Kaposi's sarcoma patients. Science. 1995;268:582–583. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials