Nerve growth factor stimulates multisite tyrosine phosphorylation and activation of the atypical protein kinase C's via a src kinase pathway - PubMed (original) (raw)
Nerve growth factor stimulates multisite tyrosine phosphorylation and activation of the atypical protein kinase C's via a src kinase pathway
M W Wooten et al. Mol Cell Biol. 2001 Dec.
Abstract
Atypical protein kinase C (PKC) isoforms are required for nerve growth factor (NGF)-initiated differentiation of PC12 cells. In the present study, we report that PKC-iota becomes tyrosine phosphorylated in the membrane coincident with activation posttreatment with nerve growth factor. Tyrosine phosphorylation and activation of PKC-iota were inhibited in a dose-dependent manner by both PP2 and K252a, src and TrkA kinase inhibitors. Purified src was observed to phosphorylate and activate PKC-iota in vitro. In PC12 cells deficient in src kinase activity, both NGF-induced tyrosine phosphorylation and activation of PKC-iota were also diminished. Furthermore, we demonstrate activation of src by NGF along with formation of a signal complex including the TrkA receptor, src, and PKC-iota. Recruitment of PKC-iota into the complex was dependent on the tyrosine phosphorylation state of PKC-iota. The association of src and PKC-iota was constitutive but was enhanced by NGF treatment, with the src homology 3 domain interacting with a PXXP sequence within the regulatory domain of PKC-iota (amino acids 98 to 114). Altogether, these findings support a role for src in regulation of PKC-iota. Tyrosine 256, 271, and 325 were identified as major sites phosphorylated by src in the catalytic domain. Y256F and Y271F mutations did not alter src-induced activation of PKC-iota, whereas the Y325F mutation significantly reduced src-induced activation of PKC-iota. The functional relevance of these mutations was tested by determining the ability of each mutant to support TRAF6 activation of NF-kappaB, with significant impairment by the Y325F PKC-iota mutant. Moreover, when the Y352F mutant was expressed in PC12 cells, NGF's ability to promote survival in serum-free media was reduced. In summary, we have identified a novel mechanism for NGF-induced activation of atypical PKC involving tyrosine phosphorylation by c-Src.
Figures
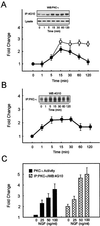
FIG. 1
NGF treatment of PC12 cells increases activation and tyrosine phosphorylation of PKC-ι. An equivalent amount of protein (500 μg) was used to determine PKC-ι activity by immune complex kinase assay, and the tyrosine phosphorylation of PKC-ι was examined by immunoprecipitation of PKC-ι followed by blotting with the 4G10 antiphosphotyrosine (anti-pTyr) antibody or immunoprecipitation (IP) of proteins containing phosphotyrosine and Western blotting with the PKC-ι antibody. The autoradiographs were scanned densitometrically and plotted to show the fold change. (A) PC12 cells were stimulated with NGF (100 ng/ml) for the indicated times. PKC-ι activity (●) and tyrosine phosphorylation of PKC-ι (○) were determined. Lysate used for immunoprecipitation was also probed with anti-PKC-ι. PKC-ι Western blots of the immunoprecipitate and the lysate are shown as insets. (B) PC12 cells were stimulated with NGF (50 ng/ml) and immunoprecipitated with the PKC-ι antibody, followed by blotting with the 4G10 anti-pTyr antibody. (C) PC12 cells were stimulated with various doses of NGF as shown for 15 min. PKC-ι activity was examined. The tyrosine phosphorylation of PKC-ι was examined by immunoprecipitation of PKC-ι followed by blotting with the 4G10 anti-pTyr antibody. The results (means ± SEM) are representative of three other independent experiments.
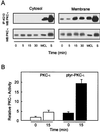
FIG. 2
NGF induces tyrosine phosphorylation and activity of membrane-associated PKC-ι. (A) Cells were stimulated with NGF (100 ng/ml) for the indicated times and subfractionated into cytosol and TX-100-soluble material (membrane). To examine NGF-mediated translocation of PKC-ι, equal amounts of protein from cell lysates were resolved by SDS-PAGE, blotted onto nitrocellulose membranes, and immunoblotted for PKC-ι. Alternatively, equal protein concentrations were immunoprecipitated (IP) with 4G10 and analyzed for PKC-ι by Western blotting (WB). WCL and S, standards of PC12 whole-cell lysates (60 μg) and purified PKC-ι, respectively, included as controls. These results are representative of two other independent experiments. (B) Activity of tyrosine-phosphorylated and non-tyrosine-phosphorylated membrane-associated PKC-ι. PC12 cells were stimulated with 100 ng of NGF/ml for 0 and 15 min. TX-100-soluble membrane material was isolated and tyrosine phosphorylated (solid bars), and non-tyrosine-phosphorylated PKC-ι (open bars) was prepared by sequential immunoprecipitation with 4G10 and anti-PKC-ι antibodies. PKC activity was determined in triplicate, and the amount of PKC in each immune complex kinase assay was normalized by Western blotting with the anti-PKC-ι antibody. These findings are means ± SEM (n = 3).
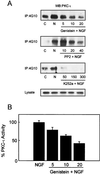
FIG. 3
Influence of kinase inhibitors on PKC-ι's tyrosine phosphorylation state and activity. (A) Cells were pretreated with genistein (0 to 20 μM), PP2 (0 to 40 μM), or K252a (0 to 300 nM) for 1 h prior to stimulation with 100 ng of NGF/ml for 15 min. Tyrosine phosphorylation of PKC-ι was determined by immunoprecipitation (IP) with 4G10 followed by PKC-ι Western blotting (WB). (B) Activity of PKC-ι was assayed in triplicate by immune complex kinase assay. PKC activity was normalized after adjusting NGF-induced activity to 100%. Values are means ± SEM (n = 3).
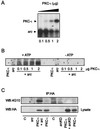
FIG. 4
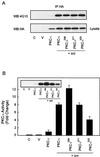
c-Src phosphorylates PKC-ι in vitro. (A) Purified c-Src was used to phosphorylate purified PKC-ι in increasing concentrations. Proteins were resolved by SDS–10% PAGE and stained, and the gel was treated for 1 h at 60°C in 1 M KOH to destroy alkali phosphate attached to Ser and Thr residues. The gel was dried and exposed to X-ray film for 1 to 2 days. Note that increasing PKC-ι phosphorylation is paralleled by decreasing src autophosphorylation. Similar results were generated in three additional experiments. (B) The experiment in panel A was replicated in the presence or absence of cold ATP in the in vitro assay. The proteins were resolved by SDS-PAGE followed by immunoblotting with the antiphosphotyrosine antibody. Increased phosphorylation by src (A) was paralleled by an increase in the tyrosine phosphorylation state of PKC-ι. (C) src, constitutively active or kinase dead, along with HA-tagged PKC-ι, was transiently coexpressed in HEK293 cells. The cell lysates were immunoprecipitated (IP) with anti-HA followed by immunoblotting with the antiphosphotyrosine antibody. Additionally, an equal amount of protein (60 μg) from whole-cell lysate (WCL) was immunoblotted with anti-HA to check for expression of PKC-ι. These results were obtained in three other identical experiments. WB, Western blotting.
FIG. 5
c-Src modulates PKC-ι activity. (A) In vitro activation of purified PKC-ι by c-Src. The PKC-specific substrate ɛ-peptide was phosphorylated in vitro by src, PKC-ι, or src-activated PKC-ι in triplicate assays, and incorporation of the 32P label into ɛ-peptide was determined by Cerenkov counting. The relative activity of PKC-ι (iota) in the presence of each of the two kinases separately or in combination is plotted. Values are means ± SEM (n = 3). (B) The levels of PKC-ι in parental and src-deficient PC12 cells were determined by Western blotting (WB) with PKC-ι antibody. (C) Parental and src-deficient PC12 cells were stimulated with 100 ng of NGF/ml for 15 min and the change in tyrosine phosphorylation of PKC-ι was determined by immunoprecipitation of tyrosine-phosphorylated proteins with 4G10 followed by PKC-ι Western blotting. The fold change in the tyrosine phosphorylation state was normalized to control for the absence of NGF. Values are means ± SEM (n = 3). (Inset) Western blots of immunoprecipitates obtained from each cell line. (C) Parental and src-deficient PC12 cells were stimulated with NGF, and PKC-ι activity was determined by immune complex kinase assay. The fold change in activity was normalized to control for the absence of NGF. Values are means ± SEM (n = 3). Values for parental and src-deficient cells are significantly different (P < 0.05).
FIG. 6
NGF treatment of PC12 cells activates c-Src and induces formation of a signal complex. (A) Time course of c-Src activation by NGF. Cells were stimulated with 100 ng of NGF/ml for the indicated times, and c-Src activity was determined by immune complex kinase assay with acid-treated enolase as the substrate. Values are means ± SEM (n = 3). (Inset) Autoradiogram of the labeled enolase. The enolase band on the autoradiograph was scanned and plotted as fold change (●). As a control, cells were treated with PP2 (40 μM; ▴) or PP3 (40 μM; ▪) prior to treatment with NGF. (B) PC12 cells were pretreated with genistein (5, 10, or 20 μM) followed by addition of NGF (100 ng/ml) for 15 min. Thereafter, TrkA was immunoprecipitated (IP), followed by Western blot (WB) analysis for PKC-ι, TrkA, and phosphotyrosine-containing TrkA as indicated.(C) TrkA, src, and PKC-ι were immunoprecipitated from NGF-stimulated cells, and immune complexes were probed for the presence of TrkA, PKC-ι, c-Src, or 4G10 as indicated. These data are representative of three other experiments.
FIG. 7
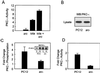
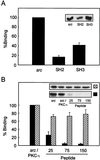
Mapping of the interaction between c-Src and PKC-ι. (A) PKC-ι was autophosphorylated and interacted in a cobinding assay with either full-length src, the src SH2 domain, or src-SH3 domain as a GST fusion construct. The data are normalized to the ratio of the moles of the proteins included in the assay. Results are presented as percent binding obtained between full-length src and PKC-ι. Values are means ± SEM (n = 3). (B) PKC-ι was phosphorylated by c-Src followed by addition, at increasing concentrations (25, 75, and 150 μM), of the PKC-ι PXXP (solid bars) or scrambled (cross-hatched bars) peptide. The resulting c-Src–PKC-ι complexes were captured using the anti-PKC-ι antibody coupled to anti-rabbit IgG-agarose. Immune complexes were resolved by SDS-PAGE and immunoblotted with the anti-c-Src antibody. As a control, purified c-Src was included at concentrations used in the cobinding assay. Results are percentages of binding of c-Src to PKC-ι, with the amount of c-src input into the assay representing 100% binding. (Insets) Autoradiographs of the respective immunoblots. These experiments were repeated three times.
FIG. 8
The effect of mutating tyrosine 256, 271, and 325 in PKC-ι on its tyrosine phosphorylation and activation. HEK293 cells were untransfected (C) or transiently transfected by calcium phosphate with HA-tagged vector pcDNA3 (V) or HA-tagged wild-type PKC-ι or Y256F, Y271F or Y325F mutants along with constitutively active src. (A) Transfected cell lysates were prepared (1 mg) and immunoprecipitated (IP) with the polyclonal anti-HA antibody followed by Western blot (WB) analysis with the PY20 antiphosphotyrosine antibody. Whole-cell lysates (lysate) were blotted (60 μg) to check for the expression of HA-tagged constructs. (B) Transfected cell lysates were prepared (500 μg), immunoprecipitated with the polyclonal HA antibody, and subjected to an immune complex kinase assay with recombinant hnRNPA1 as the substrate (2 μg). The results are the means ± SEM (n = 3).
FIG. 9
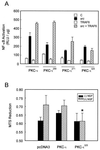
Role of PKC-ι mutants in NF-κB activation and cell survival. (A) Subconfluent cultures of HEK293 cells were transfected with 1 ng of the κB luciferase reporter gene plasmid along with PKC-ι (1 μg), src (100 ng), or TRAF6 (100 ng) alone or in combination and enough empty vector to give 2.5 μg of total DNA. After 24 h, cell extracts were prepared and the levels of luciferase activity were determined. Results are the means ± SEM of three independent experiments with duplicates. (B) PC12 cells were transfected with vector, PKC-ι or the PKC-ι Y325F mutant. The cells were switched to a serum-free environment, and cell survival was measured by MTS reduction. The PKC-ι Y325F mutant reduced NGF-dependent survival compared to either PKC-ι or vector alone (∗, P < 0.05).
FIG. 10
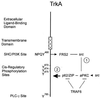
Localization of PKC-ι tyrosine phosphorylation sites. Shown is a schematic outline of the structural domains of PKC-ι including the regulatory domain, hinge region, catalytic kinase core, cysteine-rich domain (CR), and pseudosubstrate motif (PSD). Open circles, phosphorylation sites. Proteins that associate with aPKCs are listed under the region in aPKC with which they have been shown to interact: LIP (λ-interacting protein), activator of aPKCs (14); Par4, inhibitor of aPKC enzyme activity (15); tubulin (16); ZIP/p62 (44, 48); fasciculation and elongation protein zeta 1 (FEZ1) (28); ASIP (21); src (50); and cell polarity protein Par6 (23).
FIG. 11
Model for formation of TrkA–src-aPKC complexes. TrkA binds the signaling adapter FRS2/SNT at tyrosine 490, which would enable recruitment of c-src to the receptor (1) (36). On the other hand, TrkA also directly binds ZIP/p62, which recruits aPKC to the receptor (2) (67). A ternary complex between TrkA-FRS2/SNF and p62 can be formed via the binding of aPKC and src. In addition, ZIP/p62 and aPKC are capable of binding to TRAF6, the critical regulator of the NF-κB pathway, thereby directly linking components of the receptor complex to activation of the κB pathway. Therefore, the NGF-induced activation of NF-κB may be modulated by any one of the following upstream critical regulatory elements: FRS2, src, p62/ZIP, and aPKC.
Similar articles
- Mapping of atypical protein kinase C within the nerve growth factor signaling cascade: relationship to differentiation and survival of PC12 cells.
Wooten MW, Seibenhener ML, Neidigh KB, Vandenplas ML. Wooten MW, et al. Mol Cell Biol. 2000 Jul;20(13):4494-504. doi: 10.1128/MCB.20.13.4494-4504.2000. Mol Cell Biol. 2000. PMID: 10848576 Free PMC article. - Overexpression of atypical PKC in PC12 cells enhances NGF-responsiveness and survival through an NF-kappaB dependent pathway.
Wooten MW, Seibenhener ML, Zhou G, Vandenplas ML, Tan TH. Wooten MW, et al. Cell Death Differ. 1999 Aug;6(8):753-64. doi: 10.1038/sj.cdd.4400548. Cell Death Differ. 1999. PMID: 10467349 - Identification of Src as a novel atypical protein kinase C-interacting protein.
Seibenhener ML, Roehm J, White WO, Neidigh KB, Vandenplas ML, Wooten MW. Seibenhener ML, et al. Mol Cell Biol Res Commun. 1999 Jul;2(1):28-31. doi: 10.1006/mcbr.1999.0140. Mol Cell Biol Res Commun. 1999. PMID: 10527887 - The hormone-induced regulation of contact-dependent cell-cell communication by phosphorylation.
Stagg RB, Fletcher WH. Stagg RB, et al. Endocr Rev. 1990 May;11(2):302-25. doi: 10.1210/edrv-11-2-302. Endocr Rev. 1990. PMID: 2194784 Review. - Distinctive activation mechanisms and functions for protein kinase Cdelta.
Steinberg SF. Steinberg SF. Biochem J. 2004 Dec 15;384(Pt 3):449-59. doi: 10.1042/BJ20040704. Biochem J. 2004. PMID: 15491280 Free PMC article. Review.
Cited by
- NTRK3 is a potential tumor suppressor gene commonly inactivated by epigenetic mechanisms in colorectal cancer.
Luo Y, Kaz AM, Kanngurn S, Welsch P, Morris SM, Wang J, Lutterbaugh JD, Markowitz SD, Grady WM. Luo Y, et al. PLoS Genet. 2013;9(7):e1003552. doi: 10.1371/journal.pgen.1003552. Epub 2013 Jul 11. PLoS Genet. 2013. PMID: 23874207 Free PMC article. - Parameter estimate of signal transduction pathways.
Arisi I, Cattaneo A, Rosato V. Arisi I, et al. BMC Neurosci. 2006 Oct 30;7 Suppl 1(Suppl 1):S6. doi: 10.1186/1471-2202-7-S1-S6. BMC Neurosci. 2006. PMID: 17118160 Free PMC article. Review. - E3 ubiquitin ligase CHIP facilitates Toll-like receptor signaling by recruiting and polyubiquitinating Src and atypical PKC{zeta}.
Yang M, Wang C, Zhu X, Tang S, Shi L, Cao X, Chen T. Yang M, et al. J Exp Med. 2011 Sep 26;208(10):2099-112. doi: 10.1084/jem.20102667. Epub 2011 Sep 12. J Exp Med. 2011. PMID: 21911421 Free PMC article. - Multiple Gi proteins participate in nerve growth factor-induced activation of c-Jun N-terminal kinases in PC12 cells.
Tso PH, Morris CJ, Yung LY, Ip NY, Wong YH. Tso PH, et al. Neurochem Res. 2009 Jun;34(6):1101-12. doi: 10.1007/s11064-008-9880-9. Epub 2008 Nov 14. Neurochem Res. 2009. PMID: 19009346 - Regulation of polarized morphogenesis by protein kinase C iota in oncogenic epithelial spheroids.
Linch M, Sanz-Garcia M, Rosse C, Riou P, Peel N, Madsen CD, Sahai E, Downward J, Khwaja A, Dillon C, Roffey J, Cameron AJ, Parker PJ. Linch M, et al. Carcinogenesis. 2014 Feb;35(2):396-406. doi: 10.1093/carcin/bgt313. Epub 2013 Sep 26. Carcinogenesis. 2014. PMID: 24072773 Free PMC article.
References
- Abu-Amer Y, Ross F P, McHugh K P, Livolsi A, Peyron J F, Teitelbaum S L. Tumor necrosis factor alpha activation of nuclear transcription factor kappaB in marrow macrophages is mediated by c-Src tyrosine phosphorylation of IkBalpha. J Biol Chem. 1998;273:29417–29423. - PubMed
- Ahn S, Maudsley S, Luttrell L M, Lefkowitz R J, Daaka Y. Src-medicated tyrosine phosphorylation of dynamin is required for β-adrenergic receptor internalization and mitogen-activated protein kinase signaling. J Biol Chem. 1999;274:1185–1188. - PubMed
- Akiyama T, Ogawara H. Use and specificity of genistein as an inhibitor of protein-tyrosine kinases. Methods Enzymol. 1991;201:362–370. - PubMed
- Blake R A, Garcia-Paramio P, Parker P J, Courtneidge S A. Src promotes PKC-δ degradation. Cell Growth Differ. 1999;10:231–241. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous