BDCA-2, a novel plasmacytoid dendritic cell-specific type II C-type lectin, mediates antigen capture and is a potent inhibitor of interferon alpha/beta induction - PubMed (original) (raw)
. 2001 Dec 17;194(12):1823-34.
doi: 10.1084/jem.194.12.1823.
Y Sohma, J Nagafune, M Cella, M Colonna, F Facchetti, G Günther, I Johnston, A Lanzavecchia, T Nagasaka, T Okada, W Vermi, G Winkels, T Yamamoto, M Zysk, Y Yamaguchi, J Schmitz
Affiliations
- PMID: 11748283
- PMCID: PMC2193584
- DOI: 10.1084/jem.194.12.1823
BDCA-2, a novel plasmacytoid dendritic cell-specific type II C-type lectin, mediates antigen capture and is a potent inhibitor of interferon alpha/beta induction
A Dzionek et al. J Exp Med. 2001.
Abstract
Plasmacytoid dendritic cells are present in lymphoid and nonlymphoid tissue and contribute substantially to both innate and adaptive immunity. Recently, we have described several monoclonal antibodies that recognize a plasmacytoid dendritic cell-specific antigen, which we have termed BDCA-2. Molecular cloning of BDCA-2 revealed that BDCA-2 is a novel type II C-type lectin, which shows 50.7% sequence identity at the amino acid level to its putative murine ortholog, the murine dendritic cell-associated C-type lectin 2. Anti-BDCA-2 monoclonal antibodies are rapidly internalized and efficiently presented to T cells, indicating that BDCA-2 could play a role in ligand internalization and presentation. Furthermore, ligation of BDCA-2 potently suppresses induction of interferon alpha/beta production in plasmacytoid dendritic cells, presumably by a mechanism dependent on calcium mobilization and protein-tyrosine phosphorylation by src-family protein-tyrosine kinases. Inasmuch as production of interferon alpha/beta by plasmacytoid dendritic cells is considered to be a major pathophysiological factor in systemic lupus erythematosus, triggering of BDCA-2 should be evaluated as therapeutic strategy for blocking production of interferon alpha/beta in systemic lupus erythematosus patients.
Figures
Figure 4.
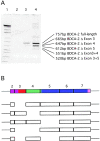
(A) Identification of putative alternative splice forms of BDCA-2 mRNA. Poly(A)+ RNA was isolated from purified PDCs and analyzed for the presence of BDCA-2 mRNA by RT-PCR amplification (lane 2, 20 PCR cycles; lane 3, 25 PCR cycles; lane 4, 30 PCR cycles). The PCR products were size-fractionated by 4–12% TBE PAGE. A further PCR amplification of individual excised bands enabled the cloning and sequencing of individual splice variants. (B) Schematic drawing of the coding region of full-length BDCA-2 mRNA. The structural domains are indicated by different colors (violet, cytoplasmic domain; red, transmembrane domain; green, neck domain; and blue, CRD) and the individual exons (exons 2–7) of the coding region are represented as boxes. Below the full-length BDCA-2 mRNA, BDCA-2 splice variants are shown with the missing exons indicated by gaps.
Figure 1.
Amino acid sequence alignment of the type II C-type lectins BDCA-2, murine dectin-2, and human DCIR. Identical or conserved residues are indicated by (*), conserved and semiconserved substitutions by (:) and (.), respectively; the putative transmembrane domains are shown in red italics; the shaded area denotes the CRD and the residues strongly conserved among C-type lectins are shown in bold type (H, hydrophobic; A, aliphatic; C, cysteine; G, glycine; E, glutamic acid; W, tryptophan; Δ, aromatic; +, involved in calcium-dependent binding of carbohydrate; +P++, so-called EPN-motif predicting mannose-type specificity [reference 46]).
Figure 2.
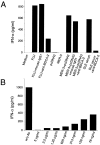
Expression analysis of BDCA-2 mRNA by RT-PCR (34 PCR-cycles) on various tissues and cell populations (1, heart; 2, brain; 3, placenta; 4, lung; 5, liver; 6, skeletal muscle; 7, kidney; 8, pancreas; 9, spleen; 10, thymus; 11, testis; 12, ovary; 13, small intestine; 14, LN; 15, bone marrow; 16, fetal liver; 17, tonsil; 18, T cells; 19, B cells; 20, NK cells; 21, monocytes; 22, CD11cbrightCD123low myeloid DCs (reference 19); 23, CD11c−CD123bright PDCs). All cDNAs were normalized using four housekeeping genes including glyceraldehyde 3-phosphate dehydrogenase (G3PDH).
Figure 3.
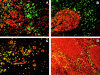
Anatomical localization of BDCA-2–expressing PDCs in inflamed tonsils. Fluorescent double staining with FITC–conjugated (green) anti–BDCA-2 mAb and Texas Red–conjugated (red) CD8 mAb (A), CD20 mAb (B), CD123 mAb (C), and anti–HLA-DR mAb (D). Note that BDCA-2–expressing PDCs are found in the T cell-rich extrafollicular areas but not within the germinal center. Like CD123, BDCA-2 is expressed on PDCs, but unlike CD123, BDCA-2 is not expressed on HEVs. One representative experiment of three is shown.
Figure 5.
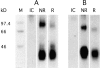
SDS-PAGE analysis of BDCA-2 immunoprecipitated from 125I-labeled PDC (A) and BDCA-2–transfected U937 human monocytoid leukemia cells (B). BDCA-2 appears as a ∼38 kD band under nonreducing (NR) and reducing (R) conditions. IC, isotype-matched control mAb. One representative experiment of three is shown.
Figure 6.
A rapid and transient rise in [Ca2+]i is induced in PDCs (left dotplots) and BDCA-2-transfected U937 cells (middle dotplots), but not in nontransfected U937 cells (right dotplots) after ligation of surface BDCA-2 with specific primary mAb (AC144, IgG1) and secondary cross-linking F(ab′)2 goat anti–mouse IgG (B). This [Ca2+]i increase is not affected when extracellular calcium is chelated with excess EGTA (C), but inhibited when src-family protein-tyrosine kinases are blocked by preincubation with the specific inhibitor PP2 (D). One representative experiment of six is shown.
Figure 7.
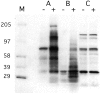
Triggering of BDCA-2 induces protein tyrosine phosphorylation in purified PDCs (A) and BDCA-2 transfected U937 cells (B), but not in BDCA-2–transfected Jurkat cells (C). Cells were incubated with medium alone (−) or with anti–BDCA-2 mAb (AC144, IgG1) (+). Cell lysates were size-fractionated by SDS-PAGE, transferred to nitrocellulose membranes, and probed with horseradish peroxidase-coupled antiphosphotyrosine mAb PY20. One representative experiment of two is shown.
Figure 8.
Presentation of anti–BDCA-2 mAb (AC144, IgG1) to a T cell clone specific for mouse IgG1 by irradiated PDCs. Anti–BDCA-2 mAb AC144 (▪) is presented more efficiently than anti-ILT3 mAb ZM3.8 (▴) and far more efficiently than anti-cytokeratin mAb CK3–11D5 (•). One representative experiment of two is shown.
Figure 9.
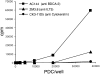
(A) Ligation of BDCA-2 suppresses induction of IFN-α/β production in PDCs. Stimulation of PDCs with FLU, anti-ss/ds DNA mAb MER-3 plus plasmid pcDNA3, or serum from a SLE patient, but not with anti-ss/ds DNA mAb MER-3 alone or plasmid pcDNA3 alone, induces production of large amounts of IFN-α/β in PDCs. Induction of IFN-α/β production with this agents can be inhibited by coincubation with anti–BDCA-2 mAb (AC144, IgG1), but not by coincubation with an isotype-matched control IgG1 mAb. The data shown are representative of more than six experiments using FLU, more than two experiments using anti-ss/ds DNA mAb MER-3 plus plasmid pcDNA3, and three experiments using SLE sera as IFN-α/β production–inducing agent. (B) Anti–BDCA-2 mAb-mediated suppression of induction of IFN-α/β production is mAb concentration dependent. Purified PDCs were coincubated with one of the IFN-α/β–inducing agents (anti-ss/ds DNA mAb MER-3 plus plasmid pcDNA3) and titrated amounts of anti–BDCA-2 mAb (AC144, IgG1). Note that concentrations below 100 ng/ml are sufficient for a 50% inhibition of the IFN-α/β response.
Comment in
- A pivotal role for the natural interferon alpha-producing cells (plasmacytoid dendritic cells) in the pathogenesis of lupus.
Rönnblom L, Alm GV. Rönnblom L, et al. J Exp Med. 2001 Dec 17;194(12):F59-63. doi: 10.1084/jem.194.12.f59. J Exp Med. 2001. PMID: 11748288 Free PMC article. Review. No abstract available.
Similar articles
- Expression of the markers BDCA-2 and BDCA-4 and production of interferon-alpha by plasmacytoid dendritic cells in systemic lupus erythematosus.
Blomberg S, Eloranta ML, Magnusson M, Alm GV, Rönnblom L. Blomberg S, et al. Arthritis Rheum. 2003 Sep;48(9):2524-32. doi: 10.1002/art.11225. Arthritis Rheum. 2003. PMID: 13130472 - Plasmacytoid dendritic cells: from specific surface markers to specific cellular functions.
Dzionek A, Inagaki Y, Okawa K, Nagafune J, Röck J, Sohma Y, Winkels G, Zysk M, Yamaguchi Y, Schmitz J. Dzionek A, et al. Hum Immunol. 2002 Dec;63(12):1133-48. doi: 10.1016/s0198-8859(02)00752-8. Hum Immunol. 2002. PMID: 12480257 Review. - CD303 (BDCA-2) signals in plasmacytoid dendritic cells via a BCR-like signalosome involving Syk, Slp65 and PLCgamma2.
Röck J, Schneider E, Grün JR, Grützkau A, Küppers R, Schmitz J, Winkels G. Röck J, et al. Eur J Immunol. 2007 Dec;37(12):3564-75. doi: 10.1002/eji.200737711. Eur J Immunol. 2007. PMID: 18022864 - BDCA-2 signaling inhibits TLR-9-agonist-induced plasmacytoid dendritic cell activation and antigen presentation.
Jähn PS, Zänker KS, Schmitz J, Dzionek A. Jähn PS, et al. Cell Immunol. 2010;265(1):15-22. doi: 10.1016/j.cellimm.2010.06.005. Epub 2010 Jul 6. Cell Immunol. 2010. PMID: 20673884 - The central role of dendritic cells and interferon-alpha in SLE.
Pascual V, Banchereau J, Palucka AK. Pascual V, et al. Curr Opin Rheumatol. 2003 Sep;15(5):548-56. doi: 10.1097/00002281-200309000-00005. Curr Opin Rheumatol. 2003. PMID: 12960479 Review.
Cited by
- The role of the vascular dendritic cell network in atherosclerosis.
Alberts-Grill N, Denning TL, Rezvan A, Jo H. Alberts-Grill N, et al. Am J Physiol Cell Physiol. 2013 Jul 1;305(1):C1-21. doi: 10.1152/ajpcell.00017.2013. Epub 2013 Apr 3. Am J Physiol Cell Physiol. 2013. PMID: 23552284 Free PMC article. Review. - Plasmacytoid dendritic cells and C1q differentially regulate inflammatory gene induction by lupus immune complexes.
Santer DM, Wiedeman AE, Teal TH, Ghosh P, Elkon KB. Santer DM, et al. J Immunol. 2012 Jan 15;188(2):902-15. doi: 10.4049/jimmunol.1102797. Epub 2011 Dec 5. J Immunol. 2012. PMID: 22147767 Free PMC article. - Plasmacytoid dendritic cells in the eye.
Jamali A, Kenyon B, Ortiz G, Abou-Slaybi A, Sendra VG, Harris DL, Hamrah P. Jamali A, et al. Prog Retin Eye Res. 2021 Jan;80:100877. doi: 10.1016/j.preteyeres.2020.100877. Epub 2020 Jul 24. Prog Retin Eye Res. 2021. PMID: 32717378 Free PMC article. Review. - Plasmacytoid Dendritic Cell Impairment in Metastatic Melanoma by Lactic Acidosis.
Monti M, Vescovi R, Consoli F, Farina D, Moratto D, Berruti A, Specchia C, Vermi W. Monti M, et al. Cancers (Basel). 2020 Jul 28;12(8):2085. doi: 10.3390/cancers12082085. Cancers (Basel). 2020. PMID: 32731406 Free PMC article. - The Dectin-2 family of C-type lectin-like receptors: an update.
Kerscher B, Willment JA, Brown GD. Kerscher B, et al. Int Immunol. 2013 May;25(5):271-7. doi: 10.1093/intimm/dxt006. Int Immunol. 2013. PMID: 23606632 Free PMC article. Review.
References
- Vollenweider, R., and K. Lennert. 1983. Plasmacytoid T-cell clusters in non-specific lymphadenitis. Virchows Arch. 44:1–14. - PubMed
- Perussia, B., V. Fanning, and G. Trinchieri. 1985. A leukocyte subset bearing HLA-DR antigens is responsible for in vitro alpha interferon production in response to viruses. Nat. Immun. Cell Growth Regul. 4:120–137. - PubMed
- Fitzgerald-Bocarsly, P., M. Feldman, M. Mendelsohn, S. Curl, and C. Lopez. 1988. Human mononuclear cells which produce interferon-alpha during NK(HSV-FS) assays are HLA-DR positive cells distinct from cytolytic natural killer effectors. J. Leukoc. Biol. 43:323–334. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous