Analysis of lung tumor initiation and progression using conditional expression of oncogenic K-ras - PubMed (original) (raw)
Analysis of lung tumor initiation and progression using conditional expression of oncogenic K-ras
E L Jackson et al. Genes Dev. 2001.
Abstract
Adenocarcinoma of the lung is the most common form of lung cancer, but the cell of origin and the stages of progression of this tumor type are not well understood. We have developed a new model of lung adenocarcinoma in mice harboring a conditionally activatable allele of oncogenic K-ras. Here we show that the use of a recombinant adenovirus expressing Cre recombinase (AdenoCre) to induce K-ras G12D expression in the lungs of mice allows control of the timing and multiplicity of tumor initiation. Through the ability to synchronize tumor initiation in these mice, we have been able to characterize the stages of tumor progression. Of particular significance, this system has led to the identification of a new cell type contributing to the development of pulmonary adenocarcinoma.
Figures
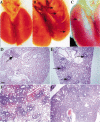
Figure 1
Viral dose dependence of tumor multiplicity. (A) Wild-type lungs 4 wk postinfection with 5 × 108 PFU of AdenoCre. The surface of the lungs is smooth and uniform. (B) Lox-K-ras G12D lungs 4 wk postinfection with 5 × 108 PFU of AdenoCre. The surface of the lungs has a cobblestone appearance; arrows indicate some of the lesions. (C) Lox-K-ras G12D lungs 10 wk postinfection with 5 × 105 PFU of AdenoCre. The surface of the lungs is smooth with the exception of a single lesion indicated by an arrow. (D) Histological sections of Lox-K-ras GD12D lungs 6 wk postinfection with 5 × 105 PFU, showing a single lesion (arrow). (E) Histological sections of Lox-K-ras GD12D lungs 6 wk postinfection with 5 × 106 PFU showing several isolated lesions (arrows). (F) Histological sections of Lox-K-ras GD12D lungs 6 wk postinfection with 5 × 107 PFU showing areas of diffuse hyperplasia. (G) Histological sections of Lox-K-ras GD12D lungs 6 wk postinfection with 5 × 108 PFU showing diffuse hyperplasia. Scale bar indicates 200 μm.
Figure 2
Immunophenotype of lesions in Lox-K-ras G12D mice. (A) CCA-positive EH in a nonrespiratory bronchiole. (B) SP-C-positive adenoma (bottom) and SP-C-negative bronchiole (top left). (C) CCA-positive papillary structures in EH continuous with an adenoma. (D) SP-C-positive papillary structures and adenoma in the same continuous lesion in the adjacent serial section. Arrows indicate the same papillary structure in C and D that is positive for both CCA and SP-C. (E) Histological section of EH continuous with an adenoma. (F) Higher magnification of papillary structures in which SP-C/CCA double-positive cells are noted by double immunofluorescence on the adjacent serial section. The box indicates the area in which the double-positive cells shown in G_–_I are located. (G) CCA immunofluorescence alone. (H) SP-C immunofluorescence alone. (I) Overlay of CCA and SP-C immunofluorescence showing the presence of cells expressing both CCA and SP-C. Scale bar indicates 50 μm in A_–_F and 25 μm in G_–_I.
Figure 3
Time-course analysis of the stages of tumor progression in Lox-K-ras G12D mice. Mice were killed at 2 (A,H), 6 (B,E,I), 12 (C,F,J), and 16 (D,G) wk postinfection; histological sections of the lungs were examined for the presence of lesions. (A) Atypical adenomatous hyperplasia in Lox-K-ras G12D lungs 2 wk postinfection. (B) Papillary adenoma 6 wk postinfection. (E) Higher magnification of the adenoma in B. (C) Large adenoma 12 wk postinfection. (F) Higher magnification of the lesion in C (arrows indicate mitosis). (D) Adenocarcinoma 16 wk postinfection. (G) Higher magnification of the lesion in D. The tumor shows increased mitotic rate (arrows), nuclear enlargement, and prominent nucleoli (field of cells indicated by asterisk). (H) EH of a respiratory bronchiole 2 wk postinfection, showing focal hyperproliferation (arrow). (I) EH of a respiratory bronchiole 6 wk postinfection, with hyperproliferative cells in the alveolar compartment. (J) EH of a respiratory (top) and terminal (bottom) bronchiole 12 wk postinfection.
References
- Campbell SL, Khosravi-Far R, Rossman KL, Clark GJ, Der CJ. Increasing complexity of Ras signaling. Oncogene. 1998;17:1395–1413. - PubMed
- Dermer GB. Origin of bronchioloalveolar carcinoma and peripheral bronchial adenocarcinoma. Cancer. 1982;49:881–887. - PubMed
- Gunning WT, Stoner GD, Goldblatt PJ. Glyceraldehyde-3-phosphate dehydrogenase and other enzymatic activity in normal mouse lung and in lung tumors. Exp Lung Res. 1991;17:255–261. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous