Induction and apoptotic regression of lung adenocarcinomas by regulation of a K-Ras transgene in the presence and absence of tumor suppressor genes - PubMed (original) (raw)
Induction and apoptotic regression of lung adenocarcinomas by regulation of a K-Ras transgene in the presence and absence of tumor suppressor genes
G H Fisher et al. Genes Dev. 2001.
Abstract
To investigate the role of an activated K-Ras gene in the initiation and maintenance of lung adenocarcinomas, we developed transgenic mice that express murine K-Ras4b(G12D) under the control of doxycycline in type II pneumocytes. Focal proliferative lesions of alveolar type II pneumocytes were observed as early as seven days after induction with doxycycline; after two months of induction, the lungs contained adenomas and adenocarcinomas, with focal invasion of the pleura at later stages. Removal of doxycycline caused a rapid fall in levels of mutant K-Ras RNA and concomitant apoptotic regression of both the early proliferative lesions and the tumors. Tumor burden was dramatically decreased by three days after withdrawal, and tumors were undetectable after one month. When similar experiments were performed with animals deficient in either the p53 gene or the Ink4A/Arf locus, tumors arose more quickly (within one month of exposure to doxycycline) and displayed more obvious histological features of malignancy; nevertheless, these tumors also regressed rapidly when the inducer was removed, implying that continued production of mutant K-Ras is necessary to maintain the viability of tumor cells in the absence as well as the presence of tumor suppressor genes. We also show that the appearance and regression of these pulmonary tumors can be readily monitored in anesthetized transgenic animals by magnetic resonance imaging.
Figures
Figure 1
Organization of transgenes and expression patterns. (A) Transgenic constructs used to generate the tetracycline operator-regulated K-Ras4bG12D (Tet-op-K-Ras4bG12D) responder mice (upper) and the Clara cell secretory protein-tet activator (CCSP-rtTA) mice (lower). The origins of the component are described in the text and their sizes are indicated in kilobases (kb). (B) RT–PCR tests for K-Ras4bG12D RNA. Total RNA was isolated from the right accessory lobe of 18 different mice and 0.25 μg was used for RT–PCR analysis of K-Ras4bG12D and actin RNAs as described in Materials and Methods. Each sample was run in parallel with a sample lacking RT. Samples were prepared from animals that did not receive doxycycline (nontransgenics, lanes 1,2; bitransgenics, lanes 3,4), from animals that received doxycycline for 9 d (bitransgenic, lane 5; responder transgene only, lane 6), and from bitransgenic mice that received doxycycline for 2 mo (lanes 7_–_9) or for 2 mo followed by withdrawal of the inducer for 3 d (lanes 10_–_14) or for 8 d (lanes 15_–_18).
Figure 2
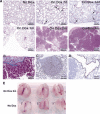
Appearance of proliferative foci and tumors after induction of mutant K-Ras with doxycycline. (A) Kinetics of induction of histological changes. Samples of lung tissue were stained with hematoxylin and eosin after exposure to inducer for 7 d, 14 d, and 1, 2, and 4 mo, as indicated. (B) Trichrome stain of tumor from a bitransgenic mouse treated for 4 mo shows invasion of stroma by tumor cells. (C, D) Cell-type-specific staining of a tumor from a mouse treated for 2 mo with doxycycline indicates properties of type II pneumocytes. Positive staining for Prosurfactant protein C (C) and negative staining of the tumor but positive staining of the airways for Clara cell secretory protein (CCSP; D). (E) Photographs of intact lungs from three bitransgenic mice induced with doxycycline for 3 mo (top row), compared to lungs from non-induced bitransgenic mice (bottom row).
Figure 3
Induction of mutant K-Ras with doxycycline promotes lung cell proliferation. The relative proportions of proliferating lung cells were determined by injection of Brdu and immunohistochemical staining after 3 h incorporation in bitransgenic mice that did not receive inducer (A), that received inducer for 14 d (B) or 2 mo (C), and that had inducer withdrawn for 3 d following 2 mo of treatment (D).
Figure 4
Effects of withdrawal of doxycycline and deinduction of mutant K-Ras on lungs of bitransgenic mice. (A) Tumor regression. Sections of lungs from bitransgenic mice treated with doxycycline for 2 mo and after removal of the inducer for 3 d, 7 d, or 1 mo were stained with H&E. (B) Appearance of apoptotic cells. Lung tumor samples were assayed for apoptotic cells using the TUNEL procedure after 2 mo on doxycycline (left) and 3 d after removal of the inducer (right). Examples of TUNEL-positive cells are indicated by red arrowheads. (C) “Pitting” on the surface of an intact lung from a bitransgenic mouse that received doxycycline for 5 mo and then had the inducer withdrawn for 3 d. The sample was fixed and dehydrated before photography.
Figure 5
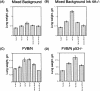
Increase and decrease in the weight of lungs from K-Ras bitransgenic mice treated with doxycycline and subjected to withdrawal of the inducer. Weights were determined after excision, fixation in 4% paraformaldehyde, and dehydration in 70% ethanol. The bitransgenic mice were from a mixed genetic background (A), a mixed background with a mutation affecting Ink4A/Arf (B), FVB/N (C), and an FVB/N background with a mutation affecting p53 (D).
Figure 6
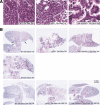
Characterization of tumors arising in K-Ras bitransgenic mice in backgrounds of or Ink4A/Arf deficiencies. (A) Malignant features of histopathology of tumors in the presence and absence of tumor suppressor genes. Samples of H&E-stained tumor-bearing lungs from indicated mouse strains after induction with doxycycline are shown at 40× magnification; for description, see text. (B) Tumors in tumor-suppressor-gene-deficient backgrounds appear more quickly during induction but regress similarly after doxycycline withdrawal. Lung slices were stained with H&E after exposure of bitransgenic mice to doxycycline for 1 or 2 mo and after withdrawal for 7 d. Bitransgenics in a wild-type background are shown on the left; in a _p53_-deficient background in the middle; and in an _Ink4A/Arf_-deficient background on the right, all as labeled.
Figure 7
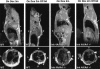
Magnetic resonance imaging of tumor regression. The pictures show sagittal (upper) and transverse (lower) images of lung fields from a bitransgenic mouse without known tumor suppressor gene mutations (left) and another bitransgenic mouse with a mutation affecting Ink4A/Arf (right). Each animal was anesthetized and subjected to MRI after induction with doxycycline for the indicated times and again after 8 d without the inducer. Arrows indicate positions of the posterior thoracic wall (upper, sagittal sections) and the outer myocardial wall (lower, transverse sections).
Similar articles
- Genetic and epigenetic alterations in lung tumors from bitransgenic Ki-rasG12C expressing mice.
Floyd HS, Jennings-Gee JE, Kock ND, Miller MS. Floyd HS, et al. Mol Carcinog. 2006 Jul;45(7):506-17. doi: 10.1002/mc.20181. Mol Carcinog. 2006. PMID: 16482519 - Tobacco smoke-induced lung tumorigenesis in mutant A/J mice with alterations in K-ras, p53, or Ink4a/Arf.
Wang Y, Zhang Z, Lubet R, You M. Wang Y, et al. Oncogene. 2005 Apr 21;24(18):3042-9. doi: 10.1038/sj.onc.1208390. Oncogene. 2005. PMID: 15846305 - Molecular comparison of human and mouse pulmonary adenocarcinomas.
Malkinson AM. Malkinson AM. Exp Lung Res. 1998 Jul-Aug;24(4):541-55. doi: 10.3109/01902149809087385. Exp Lung Res. 1998. PMID: 9659582 Review. - Genetic alterations in mouse lung tumors: implications for cancer chemoprevention.
Herzog CR, Lubet RA, You M. Herzog CR, et al. J Cell Biochem Suppl. 1997;28-29:49-63. J Cell Biochem Suppl. 1997. PMID: 9589349 Review.
Cited by
- Translation-dependent mechanisms lead to PML upregulation and mediate oncogenic K-RAS-induced cellular senescence.
Scaglioni PP, Rabellino A, Yung TM, Bernardi R, Choi S, Konstantinidou G, Nardella C, Cheng K, Pandolfi PP. Scaglioni PP, et al. EMBO Mol Med. 2012 Jul;4(7):594-602. doi: 10.1002/emmm.201200233. Epub 2012 Mar 21. EMBO Mol Med. 2012. PMID: 22359342 Free PMC article. - Sp1 expression regulates lung tumor progression.
Hsu TI, Wang MC, Chen SY, Yeh YM, Su WC, Chang WC, Hung JJ. Hsu TI, et al. Oncogene. 2012 Aug 30;31(35):3973-88. doi: 10.1038/onc.2011.568. Epub 2011 Dec 12. Oncogene. 2012. PMID: 22158040 Free PMC article. - KRAS as a Therapeutic Target.
McCormick F. McCormick F. Clin Cancer Res. 2015 Apr 15;21(8):1797-801. doi: 10.1158/1078-0432.CCR-14-2662. Clin Cancer Res. 2015. PMID: 25878360 Free PMC article. Review. - An ErbB2 splice variant lacking exon 16 drives lung carcinoma.
Smith HW, Yang L, Ling C, Walsh A, Martinez VD, Boucher J, Zuo D, Sokol ES, Pavlick DC, Frampton GM, Chmielecki J, Jones LM, Roux PP, Lockwood WW, Muller WJ. Smith HW, et al. Proc Natl Acad Sci U S A. 2020 Aug 18;117(33):20139-20148. doi: 10.1073/pnas.2007474117. Epub 2020 Jul 29. Proc Natl Acad Sci U S A. 2020. PMID: 32727899 Free PMC article. - Mutational landscape of EGFR-, MYC-, and Kras-driven genetically engineered mouse models of lung adenocarcinoma.
McFadden DG, Politi K, Bhutkar A, Chen FK, Song X, Pirun M, Santiago PM, Kim-Kiselak C, Platt JT, Lee E, Hodges E, Rosebrock AP, Bronson RT, Socci ND, Hannon GJ, Jacks T, Varmus H. McFadden DG, et al. Proc Natl Acad Sci U S A. 2016 Oct 18;113(42):E6409-E6417. doi: 10.1073/pnas.1613601113. Epub 2016 Oct 4. Proc Natl Acad Sci U S A. 2016. PMID: 27702896 Free PMC article.
References
- Bacus SS, Gudkov AV, Lowe M, Lyass L, Yung Y, Komarov AP, Keyomarsi K, Yarden Y, Seger R. Taxol-induced apoptosis depends on MAP kinase pathways (ERK and p38) and is independent of p53. Oncogene. 2001;20:147–155. - PubMed
- Berger W, Setinek U, Mohr T, Kindas-Mugge I, Vetterlein M, Dekan G, Eckersberger F, Caldas C, Micksche M. Evidence for a role of FGF-2 and FGF receptors in the proliferation of non-small cell lung cancer cells. Int J Cancer. 1999;83:415–423. - PubMed
- Budinger TF, Benaron DA, Koretsky AP. Imaging transgenic animals. Annu Rev Biomed Eng. 1999;1:611–648. - PubMed
- Cazorla M, Hernandez L, Fernandez PL, Fabra A, Peinado MA, Dasenbrock C, Tillmann T, Kamino K, Campo E, Kohler M, et al. Ki-ras gene mutations and absence of p53 gene mutations in spontaneous and urethane-induced early lung lesions in CBA/J mice. Mol Carcinog. 1998;21:251–260. - PubMed
- Chin L, Tam A, Pomerantz J, Wong M, Holash J, Bardeesy N, Shen Q, O'Hagan R, Pantginis J, Zhou H, et al. Essential role for oncogenic Ras in tumour maintenance. Nature. 1999;400:468–472. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous