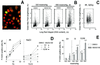Rapid activation of G2/M checkpoint after hypertonic stress in renal inner medullary epithelial (IME) cells is protective and requires p38 kinase - PubMed (original) (raw)
Rapid activation of G2/M checkpoint after hypertonic stress in renal inner medullary epithelial (IME) cells is protective and requires p38 kinase
Natalia I Dmitrieva et al. Proc Natl Acad Sci U S A. 2002.
Abstract
Cells in the kidney medulla are subject to variable and often extreme osmotic stress during concentration of the urine. Previous studies showed that renal inner medullary epithelial (IME) cells respond to hypertonicity by G(2) arrest. The purpose of the present study was to investigate the mechanisms involved in initiation and maintenance of G(2) arrest. Rapid initiation of G(2) arrest after UV radiation is mediated by p38 kinase. Here we find that p38 kinase is responsible for rapid initiation of the G(2) delay in IME cells after the hypertonic stress created by adding NaCl. High NaCl, but not high urea, rapidly initiates G(2) arrest. Inhibition of p38 kinase by SB202190 (10 microM) blocks the rapid initiation of this checkpoint both in an immortalized cell line (mIMCD3) and in second-passage IME cells from mouse renal inner medulla. p38 inhibition does not affect exit from G(2) arrest. The rapid initiation of G(2) arrest is followed by inhibition of cdc2 kinase, which is also prevented by SB202190. To assess the possible protective role of G(2) arrest, we measured DNA strand breaks as reflected by immunostaining against phospho-histone H2AX, which becomes phosphorylated on Ser-139 associated with DNA breaks. Abrogation of rapid G(2)/M checkpoint activation by SB202190 increases the histone H2AX phosphorylation in G(2)/M cells. We propose that the rapid initiation of G(2) delay by p38 kinase after hypertonicity protects the cells by decreasing the level of DNA breaks caused by aberrant mitosis entry.
Figures
Figure 1
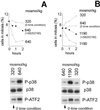
Effect of the p38 inhibitor, SB202190, on p38 abundance, phosphorylation and activity. (A) mIMCD3 cells were preincubated with SB202190 (10 μM) or DMSO for 30 min, then the media were changed for 30 min to identical ones or to otherwise identical ones with NaCl added to a total osmolality of 500 milliosmol/kg. Western analysis of p38 abundance, phosphorylation, and kinase activity. p38, total p38 protein abundance; P-p38, abundance of p38 phosphorylated on Thr-180/Tyr-182; P-ATF2, phosphorylation of the p38 substrate ATF2, as a measure of p38 kinase activity. (B) p38, activated by increasing NaCl to raise osmolality to 500 milliosmol/kg for 30 min, was immunoprecipitated as described in_Materials and Methods_, and p38 kinase activity was measured. SB202190 (10 μM) or DMSO was added to the kinase reaction. SB202190 decreases P-ATF2, confirming that it inhibits p38 kinase activity directly.
Figure 2
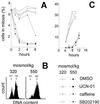
Inhibition of p38 kinase prevents hypertonicity-induced G2 checkpoint activation. (A) Representative cytogram of mIMCD3 cells at 300 milliosmol/kg, stained for P-H3 (Green Integral) and PI (DNA content). Mitotic cells have high Green Integral fluorescence because of their high level of P-H3. The area of the cytograms containing mitotic cells is bounded by the trapezoid, drawn by eye. Cells falling within this area were classified as mitotic, as confirmed by microscopy. (B) mIMCD3 cells were preincubated for 30 min with vehicle (DMSO, open symbols) or 10 μM p38 inhibitor SB202190 (closed symbols). At 0 time, the medium was replaced to add urea or NaCl to total osmolality 500 milliosmol/kg, as indicated. Cells were fixed and stained with PI for DNA content and with P-H3 antibody for identification of mitotic cells. The percent of mitotic cells was determined by laser-scanning cytometry, as shown in_A_. (C) mIMCD3 cells were preincubated with SB202190 (10 μM) or DMSO for 30 min, and then the media were changed to otherwise identical ones for 4 h (“0 h with NaCl”) or to an otherwise identical one with NaCl added to total osmolality 550 milliosmol/kg for 1, 2, or 4 h. Protein extracts were prepared, and abundance of cdc2, p38, and p38 phosphorylated on Thr-180/Tyr-182 was determined by Western blot. Also cdc2 was immunoprecipitated, and its kinase activity was measured by phosphorylation of its substrate, histone H1.
Figure 3
Effect of SB202190 (p38 inhibitor, 10 μM), UCN-01 (Chk1 inhibitor, 500 nM), or caffeine (ATM, ATR inhibitor, 2 mM) on G2 checkpoint activation and G2 exit. (A) After preincubation with DMSO or inhibitors for 30 min, the medium was changed: NaCl was added to total osmolality of 550 milliosmol/kg, as indicated. Percent of cells in mitosis was determined as in Fig. 1. (B and C) Cells were synchronized in early S by incubation for 11 h with 1 μM of the DNA polymerase inhibitor aphidicolin. Then the media were changed to media free of aphidicolin and made hypertonic by addition of NaCl to a total osmolality of 550 milliosmol/kg, as indicated. After 4 h, when G2 arrest was complete at 550 milliosmol/kg, inhibitors or DMSO were added together with nocodazole (a microtubule inhibitor, 0.5 μM) to trap all cells that entered mitosis. (B) Representative cytograms showing the position of cells in the cell cycle at the time inhibitors were added (4 h). (C) Percent of cells in mitosis, based on P-H3 staining at the indicated time points.
Figure 4
p38 activation by hypertonicity causes G2 arrest in P2mIME cells. Cells from mouse inner medullas were grown at 640 milliosmol/kg as described in Materials and Methods. Before addition of NaCl, the medium either was changed to 320 milliosmol/kg for 48 h to be more directly comparable to the experiments with mIMCD3 cells or was kept at 640 milliosmol/kg, which is more physiological for these cells. At the time of the experiments, the cells were preincubated with SB202190 (10 μM) or DMSO for 30 min, and then the media were changed to media at the same osmolality or with increased NaCl, as designated, but still containing the same DMSO or SB202190. (A and B Upper)_C_ells were fixed at indicated times and stained with P-H3 antibody for identification of mitotic cells. The percent of mitotic cells was determined by laser-scanning cytometry. (A and B Lower) Protein extracts were prepared after 30 min of elevated osmolality. p38 was analyzed by Western blot (p38, total p38 protein abundance; P-p38, phosphorylated on Thr-180/Tyr-182). p38 kinase was immunoprecipitated, and its activity is measured by phosphorylation of its substrate ATF2.
Figure 5
Abrogation of G2 checkpoint by p38 inhibition induces DNA damage with a cell cycle distribution different from that produced by ionizing radiation (IR). mIMCD3 cells were preincubated with DMSO or SB202190 (10 μM) for 30 min, then, for osmotic stress, the media were replaced for 2 or 4 h with identical ones or with otherwise identical ones to which NaCl was added to total osmolality of 550 milliosmol/kg; for ionizing radiation, cells were irradiated with indicated levels, then returned to the incubator for 20 min. Cells were stained with PI for DNA content (red) and with antiphospho-H2AX (green) to detect DNA breaks. (A) Images of cells stained with antiphospho-H2AX and PI, illustrating localized nuclear antiphospho-H2AX staining when NaCl is added in presence of SB202190. (B and C) Representative cytograms plotting maximal green fluorescence intensity (phospho-H2AX) in the nucleus vs. red fluorescence integral (DNA content). (D and E) G1, S, and G2/M cells were identified, on the basis of their DNA content and the percent of cells in each phase of cell cycle containing localized bright green nuclear fluorescence, illustrated in A, was determined by their elevated maximal green fluorescence intensity (i.e., cells in the boxes in B and C). (D) Representative experiments. (E) Analysis of H2AX phosphorylation in G1, S, and G2/M after 4 h of elevated NaCl (mean ± SEM, n = 6). *, significantly different from G1 (P < 0.01). #, significantly different from control with DMSO (P < 0.01).
Similar articles
- Initiation of a G2/M checkpoint after ultraviolet radiation requires p38 kinase.
Bulavin DV, Higashimoto Y, Popoff IJ, Gaarde WA, Basrur V, Potapova O, Appella E, Fornace AJ Jr. Bulavin DV, et al. Nature. 2001 May 3;411(6833):102-7. doi: 10.1038/35075107. Nature. 2001. PMID: 11333986 - Delta MEKK3:ER* activation induces a p38 alpha/beta 2-dependent cell cycle arrest at the G2 checkpoint.
Garner AP, Weston CR, Todd DE, Balmanno K, Cook SJ. Garner AP, et al. Oncogene. 2002 Nov 21;21(53):8089-104. doi: 10.1038/sj.onc.1206000. Oncogene. 2002. PMID: 12444545 - Protein kinase CK2 is involved in G2 arrest and apoptosis following spindle damage in epithelial cells.
Sayed M, Pelech S, Wong C, Marotta A, Salh B. Sayed M, et al. Oncogene. 2001 Oct 25;20(48):6994-7005. doi: 10.1038/sj.onc.1204894. Oncogene. 2001. PMID: 11704824 - Response of renal inner medullary epithelial cells to osmotic stress.
Burg MB. Burg MB. Comp Biochem Physiol A Mol Integr Physiol. 2002 Nov;133(3):661-6. doi: 10.1016/s1095-6433(02)00203-9. Comp Biochem Physiol A Mol Integr Physiol. 2002. PMID: 12443923 Review. - Hypertonic stress response.
Dmitrieva NI, Burg MB. Dmitrieva NI, et al. Mutat Res. 2005 Jan 6;569(1-2):65-74. doi: 10.1016/j.mrfmmm.2004.06.053. Mutat Res. 2005. PMID: 15603752 Review.
Cited by
- Osmotic Stress Interferes with DNA Damage Response and H2AX Phosphorylation in Human Keratinocytes.
Hoen L, Rudisch C, Wick M, Indenbirken D, Grundhoff A, Wegwitz F, Kalkhof S, Hildebrand J. Hoen L, et al. Cells. 2022 Mar 11;11(6):959. doi: 10.3390/cells11060959. Cells. 2022. PMID: 35326410 Free PMC article. - Nuclear P38: Roles in Physiological and Pathological Processes and Regulation of Nuclear Translocation.
Maik-Rachline G, Lifshits L, Seger R. Maik-Rachline G, et al. Int J Mol Sci. 2020 Aug 24;21(17):6102. doi: 10.3390/ijms21176102. Int J Mol Sci. 2020. PMID: 32847129 Free PMC article. Review. - p38 MAPK activity is associated with the histological degree of interstitial fibrosis in IgA nephropathy patients.
Lee J, An JN, Hwang JH, Lee H, Lee JP, Kim SG. Lee J, et al. PLoS One. 2019 Mar 21;14(3):e0213981. doi: 10.1371/journal.pone.0213981. eCollection 2019. PLoS One. 2019. PMID: 30897126 Free PMC article. - Activating PAX gene family paralogs to complement PAX5 leukemia driver mutations.
Hart MR, Anderson DJ, Porter CC, Neff T, Levin M, Horwitz MS. Hart MR, et al. PLoS Genet. 2018 Sep 14;14(9):e1007642. doi: 10.1371/journal.pgen.1007642. eCollection 2018 Sep. PLoS Genet. 2018. PMID: 30216339 Free PMC article. - Dynamic regulation of Cdr1 kinase localization and phosphorylation during osmotic stress.
Opalko HE, Moseley JB. Opalko HE, et al. J Biol Chem. 2017 Nov 10;292(45):18457-18468. doi: 10.1074/jbc.M117.793034. Epub 2017 Sep 18. J Biol Chem. 2017. PMID: 28924043 Free PMC article.
References
- Santos B C, Chevaile A, Hebert M J, Zagajeski J, Gullans S R. Am J Physiol. 1998;274:F1167–F1173. - PubMed
- Michea L, Ferguson D R, Peters E M, Andrews P M, Kirby M R, Burg M B. Am J Physiol Renal Physiol. 2000;278:F209–F218. - PubMed
- Burg M B, Kwon E D, Kültz D. Annu Rev Physiol. 1997;59:437–455. - PubMed
- Kültz D, Madhany S, Burg M B. J Biol Chem. 1998;273:13645–13651. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous