ISWI remodeling complexes in Xenopus egg extracts: identification as major chromosomal components that are regulated by INCENP-aurora B - PubMed (original) (raw)
ISWI remodeling complexes in Xenopus egg extracts: identification as major chromosomal components that are regulated by INCENP-aurora B
David E MacCallum et al. Mol Biol Cell. 2002 Jan.
Abstract
We previously characterized major components of mitotic chromosomes assembled in Xenopus laevis egg extracts and collectively referred to them as Xenopus chromosome-associated polypeptides (XCAPs). They included five subunits of the condensin complex essential for chromosome condensation. In an effort to identify novel proteins involved in this process, we have isolated XCAP-F and found it to be the Xenopus ortholog of ISWI, a chromatin remodeling ATPase. ISWI exists in two major complexes in Xenopus egg extracts. The first complex contains ACF1 and two low-molecular-weight subunits, most likely corresponding to Xenopus CHRAC. The second complex is a novel one that contains the Xenopus ortholog of the human Williams syndrome transcription factor (WSTF). In the absence of the ISWI complexes, the deposition of histones onto DNA is apparently normal, but the spacing of nucleosomes is greatly disturbed. Despite the poor spacing of nucleosomes, ISWI depletion has little effect on DNA replication, chromosome condensation or sister chromatid cohesion in the cell-free extracts. The association of ISWI with chromatin is cell cycle regulated and is under the control of the INCENP-aurora B kinase complex that phosphorylates histone H3 during mitosis. Apparently contradictory to the generally accepted model, we find that neither chromosome condensation nor chromosomal targeting of condensin is compromised when H3 phosphorylation is drastically reduced by depletion of INCENP-aurora B.
Figures
Figure 1
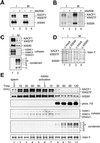
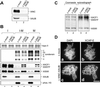
Identification of XCAP-F as the_Xenopus_ ortholog of ISWI. (A) Mitotic chromosomes were assembled from sperm chromatin in Xenopus egg mitotic HSS and purified through a sucrose cushion. Chromosomal proteins were separated by 7.5–15% SDS-PAGE and stained with Coomassie blue. (B) Mitotic HSS was fractionated by 7.5–15% SDS-PAGE and analyzed by immunoblotting with anti-XISWI antibodies against a synthetic peptide (lane 1) or a recombinant fragment (lane 2). (C) Mitotic chromosomes were assembled from sperm chromatin in mock-depleted (lanes 3 and 7) or XISWI-depleted mitotic HSS (lanes 4 and 8), and chromosome-associated polypeptides were analyzed by Coomassie blue staining (lanes 1–4) or immunoblotting with anti-XISWI antibody (lanes 5–8). As negative controls, reactions using sperm chromatin alone (lanes 1 and 5) or mitotic HSS alone (lanes 2 and 6) were processed in parallel.
Figure 2
Biochemical characterization of two ISWI complexes in Xenopus egg extracts. (A) Mitotic HSSs were incubated with preimmune (lane 1) or anti-XISWI antibody (lanes 2 and 3) in the absence (lanes 1 and 2) or presence (lane 3) of ISWI peptide. Immunoprecipitates were recovered on protein A agarose beads, washed, separated by 7.5% SDS-PAGE and stained with Coomassie blue. IgG heavy chain is indicated by IgG HC. Microsequencing of p190 and p180 identified them as XACF1 and XWSTF, respectively. The identity of three minor polypeptides (indicated by p215, p90, and p55) remains to be determined. (B) Mitotic HSS was fractionated by centrifugation through a 5–20% sucrose gradient in XBE2. Fractions were TCA-precipitated and analyzed by immunoblotting using anti-XISWI, -XACF1, and -XWSTF antibodies. The positions of three protein standards (BSA [4.6S], aldolase [7.3S] and catalase [11.4S]) are indicated. (C) Mitotic HSSs were incubated with either preimmune (lane 1), anti-XISWI (lanes 2 and 3), anti-XACF1 (lanes 4 and 5), or anti-XWSTF antibody (lanes 6 and 7) in the absence (lanes 1, 2, 4, and 6) or presence (lanes 3, 5, and 7) of competing peptides (I, ISWI peptide; A, XACF1 peptide; W, XWSTF peptide). Immunoprecipitates were recovered on protein A agarose beads, and analyzed by immunoblotting using a mixture of anti-XISWI, -XACF1, and -XWSTF antibodies (top panel). Alternatively, the samples were separated by 15% SDS-PAGE and stained with silver (bottom panel).
Figure 3
Cell cycle regulation of two ISWI complexes in Xenopus egg extracts. (A) XISWI was immunoprecipitated from interphase (lanes 1 and 2) or mitotic HSS (lanes 3 and 4) in the absence (lanes 1 and 3) or presence (lanes 2 and 4) of ISWI peptide and analyzed by immunoblotting with antibodies against XISWI, XACF1, and XWSTF. (B) XISWI was immunoprecipitated from 32P-labeled HSSs as in A, separated by SDS-PAGE and analyzed by autoradiography. (C) Chromatin was assembled in interphase (lane 1) or mitotic HSS (lane 2) and analyzed by immunoblotting with the antibodies indicated. (D) Chromatin was assembled in interphase (lanes 1 and 2) or mitotic HSS (lanes 3 and 4) that had been mock-depleted (lanes 1 and 3) or depleted of XISWI (lanes 2 and 4). Chromatin-associated polypeptides were separated by 7.5% SDS-PAGE and stained with Coomassie blue. The positions of XACF1, XWSTF, and XISWI are indicated by dots. (E) Sperm chromatin was incubated with interphase LSS. After 120 min, recombinant sea urchin cyclin BΔ90 was added to convert the extract into a mitotic state. At the indicated time points, aliquots were taken and chromatin-bound polypeptides were analyzed by immunoblotting using the antibodies indicated.
Figure 4
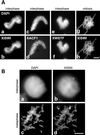
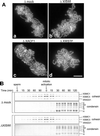
Immunolocalization of XISWI, XACF1, and XWSTF. (A) Interphase chromatin (a–f) or mitotic chromosomes (g and h) were assembled from sperm chromatin in interphase or mitotic HSS, respectively, fixed, and stained with antibodies against XISWI (b and h), XACF1 (d), or XWSTF (f). DNA was counterstained with DAPI (a, c, e, and g). Bar, 10 μm. (B) XISWI localization in _Xenopus_tissue culture cells. Interphase (a and b) or mitotic cells (c and d) were stained with an antibody against recombinant XISWI (b and d) and counterstained with DAPI (a and c). Bar, 10 μm.
Figure 5
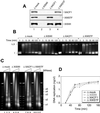
Effect of XISWI-, XACF1-, or XWSTF-depletion on interphase nuclear functions. (A) Interphase HSS was depleted with control IgG (lane 1), anti-XISWI (lane 2), anti-XACF1 (lane 3), or anti-XWSTF antibody (lane 4). Equal volumes of each HSS were analyzed by immunoblotting with the antibodies indicated. (B) Histone deposition assay. Supercoiled plasmid DNA was incubated with the control HSS (Δmock) or the depleted HSSs (ΔXISWI, ΔXACF1, and ΔXWSTF). Aliquots were taken at the time points indicated, deproteinated, separated on a 1.25% agarose gel, and stained with ethidium bromide. I, supercoiled DNA; Ir, relaxed DNA; II, nicked circular DNA. (C) Nucleosome spacing assay. Sperm chromatin was incubated with control or depleted HSS for 90 min. Each sample was then divided into three aliquots and treated with increasing concentrations of micrococcal nuclease (MNase: 0.36 U/ml, lanes 1, 4, 7, and 10; 1.2 U/ml, lanes 2, 5, 8, and 11; and 3.6 U/ml, lanes 3, 6, 9, and 12). After deproteination, the DNA was separated on a 1.25% agarose gel and stained with ethidium bromide. A 100-base pair ladder was used as a molecular weight marker. The positions of nucleosomal oligomers are indicated. (D) DNA replication assay. Sperm chromatin was incubated with control or depleted LSS in the presence of [α-32P]dATP. Aliquots were taken at the time points indicated, deproteinated, precipitated with TCA, and placed on a filter for scintillation counting. The extent of replication is expressed as nanograms of DNA replicated per microliter of extract.
Figure 6
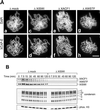
Effect of XISWI-, XACF1-, or XWSTF-depletion on chromosome condensation. (A) Sperm chromatin was incubated in mitotic HSS that had been mock-depleted (a and b) or depleted of XISWI (c and d), XACF1 (e and f) or XWSTF (g and h) for 2 h. Chromosomes were fixed and stained with DAPI (a, c, e, and g) and anti–XCAP-E antibody (b, d, f, and h). Bar, 10 μm. (B) Sperm chromatin was incubated with mock-depleted (Δmock) or XISWI-depleted (ΔXISWI) mitotic HSS. Aliquots were taken at the times indicated, and chromatin-bound polypeptides were analyzed by immunoblotting.
Figure 7
Effect of XISWI-, XACF1-, or XWSTF-depletion on sister chromatid cohesion. (A) Sperm chromatin was incubated for 2 h with interphase LSS that had been mock-depleted (a) or depleted of XISWI (b), XACF1 (c) or XWSTF (d), and half a volume of the corresponding mitotic LSS was added to drive the extract into mitosis. After incubation for 90 min, chromosomes were fixed and stained with anti–XCAP-E antibody. Bar, 10 μm. (B) Sperm chromatin was incubated with either mock-depleted (Δmock) or XISWI-depleted (ΔXISWI) LSS. After 120 min, recombinant sea urchin cyclin B Δ90 was added to drive the extract into mitosis. Aliquots were taken at the indicated time points, and chromatin was purified and analyzed by immunoblotting with antibodies against cohesin and condensin subunits.
Figure 8
Effects of immunodepletion of INCENP (XINC) and aurora B (XAUB) from Xenopus egg extracts. (A) Mitotic HSS was depleted either with control IgG, (lane 1) or with a mixture of antibodies against XINC and XAUB (lane 2) and analyzed by immunoblotting using anti-XINC (top panel) or anti-XAUB antibody (bottom panel). (B) Chromatin was assembled in either interphase (lanes 1 and 2) or mitotic HSS (lanes 5 and 6). Alternatively, chromatin was first assembled in interphase HSS, which was then driven into mitosis by addition of half a volume of mitotic HSS (lanes 3 and 4). In each case, the HSSs had been mock-depleted (lanes 1, 3, and 5) or depleted of XINC and XAUB (lanes 2, 4, and 6). After incubation, chromatin was isolated and associated polypeptides were analyzed by immunoblotting using the antibodies indicated. (C) Mitotic HSS was mock-depleted (lanes 1 and 3) or depleted of XINC and XAUB (lanes 2 and 4) and labeled by addition of [γ-32P]ATP. The XISWI complexes were immunoprecipitated with anti-XACF1 and anti-XWSTF antibodies, fractionated by SDS-PAGE, and analyzed by Coomassie blue staining (lanes 1 and 2) or autoradiography (lane 3 and 4). (D) Sperm chromatin was incubated with mitotic HSS that had been mock-depleted (a and b) or depleted of XINC and XAUB (c and d). The assembled chromosomes were stained with DAPI (a and c) or anti–XCAP-E antibody (b and d). Bar, 10 μm.
Similar articles
- Cohesin release is required for sister chromatid resolution, but not for condensin-mediated compaction, at the onset of mitosis.
Losada A, Hirano M, Hirano T. Losada A, et al. Genes Dev. 2002 Dec 1;16(23):3004-16. doi: 10.1101/gad.249202. Genes Dev. 2002. PMID: 12464631 Free PMC article. - Analysis of the role of Aurora B on the chromosomal targeting of condensin I.
Takemoto A, Murayama A, Katano M, Urano T, Furukawa K, Yokoyama S, Yanagisawa J, Hanaoka F, Kimura K. Takemoto A, et al. Nucleic Acids Res. 2007;35(7):2403-12. doi: 10.1093/nar/gkm157. Epub 2007 Mar 28. Nucleic Acids Res. 2007. PMID: 17392339 Free PMC article. - A human condensin complex containing hCAP-C-hCAP-E and CNAP1, a homolog of Xenopus XCAP-D2, colocalizes with phosphorylated histone H3 during the early stage of mitotic chromosome condensation.
Schmiesing JA, Gregson HC, Zhou S, Yokomori K. Schmiesing JA, et al. Mol Cell Biol. 2000 Sep;20(18):6996-7006. doi: 10.1128/MCB.20.18.6996-7006.2000. Mol Cell Biol. 2000. PMID: 10958694 Free PMC article. - Three-step model for condensin activation during mitotic chromosome condensation.
Bazile F, St-Pierre J, D'Amours D. Bazile F, et al. Cell Cycle. 2010 Aug 15;9(16):3243-55. doi: 10.4161/cc.9.16.12620. Epub 2010 Aug 7. Cell Cycle. 2010. PMID: 20703077 Review. - Chromosomal passengers: the four-dimensional regulation of mitotic events.
Vagnarelli P, Earnshaw WC. Vagnarelli P, et al. Chromosoma. 2004 Nov;113(5):211-22. doi: 10.1007/s00412-004-0307-3. Epub 2004 Sep 4. Chromosoma. 2004. PMID: 15351889 Review.
Cited by
- Chromosome structure deficiencies in MCPH1 syndrome.
Arroyo M, Trimborn M, Sánchez A, Hirano T, Neitzel H, Marchal JA. Arroyo M, et al. Chromosoma. 2015 Dec;124(4):491-501. doi: 10.1007/s00412-015-0512-2. Epub 2015 Apr 7. Chromosoma. 2015. PMID: 25845520 - Reconstitution of mitotic chromatids with a minimum set of purified factors.
Shintomi K, Takahashi TS, Hirano T. Shintomi K, et al. Nat Cell Biol. 2015 Aug;17(8):1014-23. doi: 10.1038/ncb3187. Epub 2015 Jun 15. Nat Cell Biol. 2015. PMID: 26075356 - ISWI is a RanGTP-dependent MAP required for chromosome segregation.
Yokoyama H, Rybina S, Santarella-Mellwig R, Mattaj IW, Karsenti E. Yokoyama H, et al. J Cell Biol. 2009 Dec 14;187(6):813-29. doi: 10.1083/jcb.200906020. J Cell Biol. 2009. PMID: 20008562 Free PMC article. - Cohesin regulation: fashionable ways to wear a ring.
Losada A. Losada A. Chromosoma. 2007 Aug;116(4):321-9. doi: 10.1007/s00412-007-0104-x. Epub 2007 Mar 1. Chromosoma. 2007. PMID: 17333234 Review. - A genetic screen for functional partners of condensin in fission yeast.
Robellet X, Fauque L, Legros P, Mollereau E, Janczarski S, Parrinello H, Desvignes JP, Thevenin M, Bernard P. Robellet X, et al. G3 (Bethesda). 2014 Feb 19;4(2):373-81. doi: 10.1534/g3.113.009621. G3 (Bethesda). 2014. PMID: 24362309 Free PMC article.
References
- Aalfs JD, Kingston RE. What does ‘chromatin remodeling’ mean? Trends Biochem Sci. 2000;25:548–555. - PubMed
- Adachi Y, Luke M, Laemmli UK. Chromosome assembly in vitro: topoisomerase II is required for condensation. Cell. 1991;64:137–148. - PubMed
- Adams RR, Wheatley SP, Gouldsworthy AM, Kandels-Lewis SE, Carmena M, Smythe C, Gerloff DL, Earnshaw WC. INCENP binds the Aurora-related kinase AIRK2 and is required to target it to chromosomes, the central spindle and cleavage furrow. Curr Biol. 2000;10:1075–1078. - PubMed
- Bell SP, Kobayashi R, Stillman B. Yeast origin recognition complex functions in transcription silencing and DNA replication. Science. 1993;262:1844–1849. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous