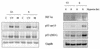Hypoxia links ATR and p53 through replication arrest - PubMed (original) (raw)
Hypoxia links ATR and p53 through replication arrest
Ester M Hammond et al. Mol Cell Biol. 2002 Mar.
Abstract
Previous studies have demonstrated that phosphorylation of human p53 on serine 15 contributes to protein stabilization after DNA damage and that this is mediated by the ATM family of kinases. However, cellular exposure to hypoxia does not induce any detectable level of DNA lesions compared to ionizing radiation, and the oxygen dependency of p53 protein accumulation differs from that of HIF-1, the hypoxia-inducible transcription factor. Here we show that, under severe hypoxic conditions, p53 protein accumulates only in S phase and this accumulation correlates with replication arrest. Inhibition of ATR kinase activity substantially reduces hypoxia-induced phosphorylation of p53 protein on serine 15 as well as p53 protein accumulation. Thus, hypoxia-induced cell growth arrest is tightly linked to an ATR-signaling pathway that is required for p53 modification and accumulation. These studies indicate that the ATR kinase plays an important role during tumor development in responding to hypoxia-induced replication arrest, and hypoxic conditions could select for the loss of key components of ATR-dependent checkpoint controls.
Figures
FIG. 1.
In RKO cells HIF-1α is induced at oxygen concentrations of 2 and 0.02%, while p53 is stabilized only at 0.02%. (A) RKO cells were grown in glass dishes at the oxygen concentrations indicated (20, 2, or 0.02%) and harvested after 6, 12, and 24 h. The total levels of HIF-1α, p53 and Gapdh are shown. (B) RKO cells were grown as indicated for panel A. Growth at 0.02% oxygen induced a decrease in DNA synthesis as measured by [3H]thymidine incorporation. In contrast, cells grown in normal (20% O2) and 2% O2 concentrations continued to cycle and have similar profiles. (C) The cell lines shown were grown in glass dishes and treated with hypoxia for the indicated time periods. Shown are the expression levels of hexokinase I, RTP, adrenomedullin, NIP3, and adipophilin which were determined by hybridization to an oligo-based Affymetrix microarray.
FIG. 2.
Hypoxia induces p53 stabilization and phosphorylation in S-phase cells rather than cells in G1. Mitotic RKO cells were harvested by shaking flasks of exponentially growing populations in order to loosen adhering cells. After shake-off, cells were plated in glass dishes and placed into a hypoxia chamber for 8 h. Greater than 90% of these cells are in the G1 phase of the cell cycle, which is approximately 8 h for RKO cells. Cells were plated and allowed to grow under normoxic conditions for 8 to 10 h after shake-off to produce a synchronized S-phase population. These cells were then placed in the hypoxia chamber for 8 h. Synchronized populations of G1- or S-phase cells were also treated with γIR (5 h after treatment with 8 Gy) and UV (5 h after treatment with 50 J m−2). The levels of p53 (total), p53 serine 15, and Gapdh were determined by Western blotting. The levels of HIF-1α protein are also shown for the hypoxia-treated samples.
FIG. 3.
p53 is phosphorylated at residues serine 15 and 37 in response to hypoxia. Total cell extracts were prepared from RKO (A) and 293T (B) cells, which had been grown in glass dishes. Cells were treated with hypoxia, DFO (100 μM; 24 h), CoCl2 (150 μM; 24 h) or Hu (1.5 mM; 24 h). Immunoblots were carried out to show the levels of HIF-1α, total p53, p53 serine 15, p53 serine 37, and Gapdh.
FIG. 4.
The phosphorylation of p53 at Serine 15 in response to hypoxia is independent of ATM. GM1526 ATM−/− and GM536 ATM+/+ were exposed to either hypoxia or 6 Gy of γIR. The lack of ATM had little or no effect on the levels of p53 serine 15 in response to hypoxia. In contrast, there was threefold less p53 serine 15 in the ATM-null cells after γIR than in the ATM wild-type cells. The relative increases in signal were determined by using a phosphoimager.
FIG. 5.
ATR is responsible for phosphorylating p53 at serine 15 during hypoxia exposure. (A) 293T cells were transiently transfected with either a β-Gal or an ATR kinase-dead construct. Cells were treated with either hypoxia or γIR (4 h after treatment with 6 Gy), and the Western blotting experiments shown were carried out. (B) RKO cells were transiently transfected with either a β-Gal or an ATR kinase-dead construct. After transfection, cells were treated with hypoxia for 24 h and the Western blotting experiments shown were carried out.
FIG. 6.
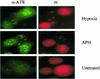
ATR forms distinct nuclear foci in hypoxic cells. RKO cells were grown on chamber slides and incubated in hypoxic conditions for 18 h. As a positive control RKOs were also treated with APH for 24 h (5 μg ml−1). After treatment, cells were fixed and permeabilized before staining with protein G-purified α-ATR (49). Slides were counterstained with propidium iodide to demonstrate that the foci seen were nuclear.
FIG. 7.
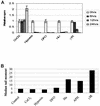
Hypoxia induces a rapid intra-S-phase arrest in the absence of any detectable DNA damage. RKO cells were treated with a variety of stresses, and a [3H]thymidine incorporation assay was carried out. The treatments were as follows: hypoxia (0.02% oxygen), 1.5 mM Hu, 8 Gy γIR, 100 μm DFO, and 150 μM CoCl2 for the times indicated. Comet assays were carried out to determine relative amounts of damage caused after each treatment. The median tail moment for each stress is shown. There was no increase in DNA damage over control levels after 24 h of hypoxia or CoCl2 treatment and only a slight increase with DFO. All the other stresses used have DNA damage associated with them to various degrees.
FIG. 8.
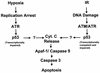
Model for the accumulation and activation of p53 by hypoxia and irradiation. Many genotoxic stresses lead to the accumulation of p53. These stresses can be divided into three groups: those that induce a replication arrest (hypoxia), those that induce DNA damage (IR and chemotherapeutic drugs), and those that do both (Hu and APH). See text for further details.
References
- Alarcon, R., C. Koumenis, R. K. Geyer, C. G. Maki, and A. J. Giaccia. 1999. Hypoxia induces p53 accumulation through MDM2 down-regulation and inhibition of E6-mediated degradation. Cancer Res. 59:6046-6051. - PubMed
- An, W. G., M. Kanekal, M. C. Simon, E. Maltepe, M. V. Blagosklonny, and L. M. Neckers. 1998. Stabilization of wild-type p53 by hypoxia-inducible factor 1alpha. Nature 392:405-408. - PubMed
- Banin, S., L. Moyal, S. Shieh, Y. Taya, C. W. Anderson, L. Chessa, N. I. Smorodinsky, C. Prives, Y. Reiss, Y. Shiloh, and Y. Ziv. 1998. Enhanced phosphorylation of p53 by ATM in response to DNA damage. Science 281:1674-1677. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- P01 CA067166/CA/NCI NIH HHS/United States
- CA 67166/CA/NCI NIH HHS/United States
- CA 88480/CA/NCI NIH HHS/United States
- R01 CA088480/CA/NCI NIH HHS/United States
- R37 CA088480/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous