Targeted point mutations of p53 lead to dominant-negative inhibition of wild-type p53 function - PubMed (original) (raw)
Targeted point mutations of p53 lead to dominant-negative inhibition of wild-type p53 function
Annemieke de Vries et al. Proc Natl Acad Sci U S A. 2002.
Abstract
The p53 tumor suppressor gene is the most frequently mutated gene in human cancers, and germ-line p53 mutations cause a familial predisposition for cancer. Germ-line or sporadic p53 mutations are usually missense and typically affect the central DNA-binding domain of the protein. Because p53 functions as a tetrameric transcription factor, mutant p53 is thought to inhibit the function of wild-type p53 protein. Here, we studied the possible dominant-negative inhibition of wild-type p53 protein by two different, frequently occurring point mutations. The R270H and P275S mutations were targeted into the genome of mouse embryonic stem cells to allow the analysis of the effects of the mutant proteins expressed in normal cells at single-copy levels. In embryonic stem cells, the presence of a heterozygous point-mutated allele resulted in delayed transcriptional activation of several p53 downstream target genes on exposure to gamma irradiation. Doxorubicin-induced apoptosis was severely affected in the mutant embryonic stem cells compared with wild-type cells. Heterozygous mutant thymocytes had a severe defect in p53-dependent apoptotic pathways after treatment with gamma irradiation or doxorubicin, whereas p53-independent apoptotic pathways were intact. Together these data demonstrate that physiological expression of point-mutated p53 can strongly limit overall cellular p53 function, supporting the dominant-negative action of such mutants. Also, cells heterozygous for such mutations may be compromised in terms of tumor suppression and response to chemotherapeutic agents.
Figures
Figure 1
Generation of p53 point mutated alleles in ES cells. Scheme for targeting one allele of the murine p53 gene in ES cells with a targeting vector containing either the R270H or the P275S mutation (asterisk), and a neo-TK selectable marker cassette flanked by LoxP sites (first selection round). Homologous integration of the vector results in an additional _Eco_RI site (situated in the promoter of pMC-neo), which is used for Southern blot analysis of neomycin-resistant ES cell clones. In the homologous recombinant clones excision of the neo-TK selectable marker cassette was accomplished by transfection with circular pMC-CreN plasmid (second selection round). The resulting allele differs from the wild-type allele, besides the R270H or P275S mutation, only in the presence of one LoxP site downstream of the coding sequences.
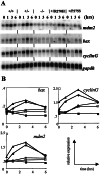
Figure 2
Expression levels of p53 target genes in ES cells upon treatment with γ irradiation. (A) Wild-type D3 (+/+);p53+/− (+/−);p53_−/− (−/−);p53+/R270H (+/R270H); and_p53+/P275S (+/P275S) ES cells were grown for 2 days, and exposed to a single dose of γ irradiation (500 cGy). At 1, 3, and 6 hr after the treatment, RNA was isolated. Northern blots were probed with bax, cyclinG,mdm2, and gapdh cDNA probes. The 0-hr time points represent untreated cells, isolated at the same time as the 3-hr time point after γ irradiation. (B) Quantitation of Northern blot signals normalized for expression levels of_gapdh_ (used as a loading control). Note that in the_bax_ graph, the scale of the y axis is different from the other axes. ▴, Wild-type D3; ●, p53+/−; ■, _p53_−/−; ○,p53+/R270H; □,p53+/P275S.
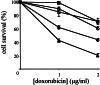
Figure 3
Doxorubicin-induced apoptosis in ES cells. Wild-type D3 (▴), p53+/−(●), _p53_−/−(■), p53+/R270H (○), and p53+/P275S (□) ES cells were grown to subconfluency, and exposed to either 1 or 2 μg/ml doxorubicin in the culture medium. After 24 hr, the cells were stained with PI and annexin V antibody, and the numbers of viable cells were determined by fluorescence-activated cell sorter analysis. Data are averages of four independent experiments.
Figure 4
The effect of heterozygous point mutations in p53 on apoptosis in thymocytes. (A) _p53_-dependent and -independent apoptosis. Thymocytes of mice of all different genotypes were isolated (▴, wild type; ●, p53+/−; ■, p53_−/−; ○,p53+/R270H; □,p53+/P275S) and exposed in vitro to γ irradiation (500 cGy), doxorubicin (0.2 μg/ml), or dexamethasone (1 μM). At the time points indicated, thymocytes were stained with annexin V and PI. The relative percentage of viable cells (negative for both PI and annexin V) for each sample is shown. All values are normalized to the number of cells remaining viable in untreated cultures derived from the same animal stained simultaneously. Data are representatives of ≥2 independent experiments (i.e., mice). (B) Northern blot of p53 target genes in thymocytes after γ irradiation (500 cGy). Two or 5 hr after the treatment, RNA was isolated, and Northern blots were probed with_bax, cyclinG, and _gapdh_cDNA probes. (C) Quantitation of Northern blot signals normalized for expression levels of gapdh (used as a loading control). ▴, Wild type; ●,p53+/−; ■,_p53_−/−; ○,p53+/R27OH; and □,p53+/P275S.
Similar articles
- Knock-in mice with a chimeric human/murine p53 gene develop normally and show wild-type p53 responses to DNA damaging agents: a new biomedical research tool.
Luo JL, Yang Q, Tong WM, Hergenhahn M, Wang ZQ, Hollstein M. Luo JL, et al. Oncogene. 2001 Jan 18;20(3):320-8. doi: 10.1038/sj.onc.1204080. Oncogene. 2001. PMID: 11313961 - A transcriptional activation function of p53 is dispensable for and inhibitory of its apoptotic function.
Kokontis JM, Wagner AJ, O'Leary M, Liao S, Hay N. Kokontis JM, et al. Oncogene. 2001 Feb 8;20(6):659-68. doi: 10.1038/sj.onc.1204139. Oncogene. 2001. PMID: 11313999 - Oncogenic mutations of the p53 tumor suppressor: the demons of the guardian of the genome.
Sigal A, Rotter V. Sigal A, et al. Cancer Res. 2000 Dec 15;60(24):6788-93. Cancer Res. 2000. PMID: 11156366 Review. - [p53].
Takahashi R. Takahashi R. Gan To Kagaku Ryoho. 1997 Sep;24(11):1381-5. Gan To Kagaku Ryoho. 1997. PMID: 9309129 Review. Japanese.
Cited by
- Current insights and future directions of Li-Fraumeni syndrome.
Hosseini MS. Hosseini MS. Discov Oncol. 2024 Oct 15;15(1):561. doi: 10.1007/s12672-024-01435-w. Discov Oncol. 2024. PMID: 39404911 Free PMC article. Review. - p53 at the crossroads of tumor immunity.
Efe G, Rustgi AK, Prives C. Efe G, et al. Nat Cancer. 2024 Jul;5(7):983-995. doi: 10.1038/s43018-024-00796-z. Epub 2024 Jul 15. Nat Cancer. 2024. PMID: 39009816 Review. - Cell Competition Eliminates Aneuploid Human Pluripotent Stem Cells.
Ya A, Deng C, Godek KM. Ya A, et al. bioRxiv [Preprint]. 2024 May 10:2024.05.08.593217. doi: 10.1101/2024.05.08.593217. bioRxiv. 2024. PMID: 38766106 Free PMC article. Preprint. - Anticancer Therapeutic Strategies Targeting p53 Aggregation.
Ferretti GDS, Quarti J, Dos Santos G, Rangel LP, Silva JL. Ferretti GDS, et al. Int J Mol Sci. 2022 Sep 20;23(19):11023. doi: 10.3390/ijms231911023. Int J Mol Sci. 2022. PMID: 36232329 Free PMC article. Review. - A p53 transcriptional signature in primary and metastatic cancers derived using machine learning.
Keshavarz-Rahaghi F, Pleasance E, Kolisnik T, Jones SJM. Keshavarz-Rahaghi F, et al. Front Genet. 2022 Aug 29;13:987238. doi: 10.3389/fgene.2022.987238. eCollection 2022. Front Genet. 2022. PMID: 36134028 Free PMC article.
References
- Levine A J. Cell. 1997;88:323–331. - PubMed
- Cox L S, Lane D P. BioEssays. 1995;17:501–508. - PubMed
- Gottlieb T M, Oren M. Biochim Biophys Acta. 1996;1287:77–102. - PubMed
- Ko L J, Prives C. Genes Dev. 1996;10:1054–1072. - PubMed
- Lowe S W, Bodis S, McClatchey A, Remington L, Ruley H E, Fisher D E, Housman D E, Jacks T. Science. 1994;266:807–810. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous