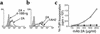Provision of antigen and CD137 signaling breaks immunological ignorance, promoting regression of poorly immunogenic tumors - PubMed (original) (raw)
Provision of antigen and CD137 signaling breaks immunological ignorance, promoting regression of poorly immunogenic tumors
Ryan A Wilcox et al. J Clin Invest. 2002 Mar.
Abstract
Treatment of advanced, poorly immunogenic tumors in animal models, considered the closest simulation available thus far for conditions observed in cancer patients, remains a major challenge for cancer immunotherapy. We reported previously that established tumors in mice receiving an agonistic mAb to the T cell costimulatory molecule 4-1BB (CD137) regress due to enhanced tumor antigen-specific cytotoxic T lymphocyte responses. In this study, we demonstrate that several poorly immunogenic tumors, including C3 tumor, TC-1 lung carcinoma, and B16-F10 melanoma, once established as solid tumors or metastases, are refractory to treatment by anti-4-1BB mAb. We provide evidence that immunological ignorance, rather than anergy or deletion, of tumor antigen--specific CTLs during the progressive growth of tumors prevents costimulation by anti-4-1BB mAb. Breaking CTL ignorance by immunization with a tumor antigen-derived peptide, although insufficient to stimulate a curative CTL response, is necessary for anti--4-1BB mAb to induce a CTL response leading to the regression of established tumors. Our results suggest a new approach for immunotherapy of human cancers.
Figures
Figure 1
mAb 2A binds murine 4-1BB and costimulates T cell growth in vitro. (a) Nylon wool–purified T cells stimulated for 24 hours in the presence of plate-bound anti-CD3 and anti-CD28. Cells were stained with mAb 2A or an isotype control (shaded region), both in the presence and absence of 4-1BB–Ig. (b) Cultured S49.1 murine T lymphoma cells were stained with mAb 2A or a commercially available mAb against 4-1BB (clone 1AH2; PharMingen). (c) Nylon wool–purified T cells were stimulated with a suboptimal dose of plate-bound anti-CD3 (0.1 μg/ml) and the indicated concentrations of plate-bound rat IgG (filled circles) or mAb 2A (open circles). 3H-TdR, 3H–tritium deoxyribonucleotide.
Figure 2
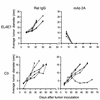
Treatment of established EL4E7 and C3 tumors with mAb 2A. Mice were injected subcutaneously with 1 × 106 C3 cells (bottom panel) or 4 × 106 EL4E7 cells (top panel). Seven and ten days later, mice were given 100 μg of either control rat IgG (left) or mAb 2A (right). Tumor size was assessed weekly and is reported as the average of two perpendicular diameters. Tumors that were no longer palpable were considered to have regressed. Each experiment is representative of at least three similarly performed experiments.
Figure 3
Cytolytic activity of tumor-specific CTLs in the TDLNs of C3- and EL4E7-bearing mice. Mice were injected subcutaneously with 1 × 106 C3 cells or 4 × 106 EL4E7 cells on day 0. On days 1 and 4 they were administered 100 μg of either control rat IgG or mAb 2A. Seven days later, TDLNs were harvested and restimulated in vitro with irradiated C3 or EL4E7 cells for 4 days. After 4 days in culture, cells were used as effectors in a 4-hour 51Cr release assay against EL4, EL4E7, RMA-S, E7 peptide–pulsed RMA-S, or C3 target cells at the indicated effector/target (E/T) ratios. Effectors were generated from both C3-draining LNs (a) and EL4E7-draining LNs (b) of mice treated with the control rat IgG (filled circles) or mAb 2A (open circles). TDLNs from two to three mice were pooled in each of the experiments. Data shown are representative of three experiments. (c) An E7-specific CTL line generated by repeated in vitro stimulation of LN cells from an E7 peptide–immunized B6 mouse was used as the effector in a 4-hour 51Cr release assay against EL4 cells, E7 peptide–pulsed EL4 cells, or C3 target cells at the indicated E/T ratios.
Figure 4
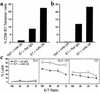
Frequency of E7-specific CTLs in the TDLNs of C3- and EL4E7-bearing mice. TDLNs were harvested from tumor-bearing mice and restimulated in vitro as described in Figure 3 legend. After 4 days in culture, cells were double-stained with tetrameric H-2Db–E7 or H-2Db–Vp2 (aa 121–130) and anti-CD8. The percentage of total CD8+ cells that stained with the H-2Db–E7 tetramer is shown (a). Viable CD8+ cells were gated, and the fraction of cells that stained with tetrameric H-2Db–E7 (aa 49–57) is shown. Data are presented as the average of three independent experiments (b). Background staining with the H-2Db–Vp2 tetramer was subtracted from the original data.
Figure 5
Frequency of E7-specific CTLs in peptide-immunized mice. Mice were injected subcutaneously with 1 × 106 C3 cells. Seven days later, tumor-bearing mice (a) or naive mice (b) were immunized intradermally on the contralateral flank with 50 μg of E7 (aa 49–57) peptide emulsified in IFA (Sigma Chemical Co.). Ab’s including the control rat IgG and mAb 2A (100 μg) were given intraperitoneally in 0.5 ml PBS on days 1 and 4. Cells from two to three mice in each group were pooled. Seven days later, LN cells draining the site of immunization were stained with H-2Db–E7 following in vitro restimulation with irradiated C3 cells, as previously described. (c) After the 4-day restimulation, cells from the draining LNs of the naive mice immunized with E7 peptide and given control rat IgG (filled circles) or mAb 2A (open circles) were used as effectors in a 4-hour 51Cr release assay against EL4 (left panel), E7 peptide–pulsed EL4 (middle panel), and C3 (right panel) targets at the indicated E/T ratios. Data shown is representative of at least four experiments.
Figure 6
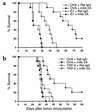
Regression of established C3 tumors after treatment with the E7 peptide and mAb 2A. Mice were injected subcutaneously with 1 × 106 C3 cells. Seven days later, mice were divided into two groups and were immunized intradermally with either Vp2 or E7 peptide (50 μg/mouse) emulsified in IFA. On days 7 and 10, mice received 100 μg of the control rat IgG or mAb 2A intraperitoneally. Tumor size was assessed weekly for each mouse, and the mice were sacrificed at the times indicated, when the average tumor diameter reached 15 mm (filled circles). Mice bearing tumors less than an average of 15 mm in diameter were sacrificed upon termination of the experiment, at 13 weeks (open circles). Data was combined from two similarly performed experiments. The improved survival observed in the treatment group receiving both the E7 peptide and mAb 2A was statistically significant, as determined by the χ2 test. (P ≤ 0.01).
Figure 7
COPP treatment of established TC-1 and B16-F10 lung metastases. Mice were given 1 × 104 TC-1 cells (a) or 1 × 105 B16-F10 cells (b) intravenously and were immunized intradermally 3 days later with the OVA peptide (circles), the E7 peptide (triangles in a), or the trp-2 peptide (triangles in b). The peptides were emulsified in IFA and the mice were given 50 μg of peptide at each of two immunization sites. On the day of immunization, and again 3 days later, the mice received 100 μg of either rat IgG or mAb 2A intraperitoneally. The mice were monitored daily for the duration of the experiment. Survival data from two identically performed experiments (n = 10) were combined. Mice treated with the E7 peptide (in a) or trp-2 peptide (in b) plus mAb 2A had a significant survival advantage in all experiments performed, as determined by the log rank test (P ≤ 0.001).
Similar articles
- Divergent effects of 4-1BB antibodies on antitumor immunity and on tumor-reactive T-cell generation.
Kim JA, Averbook BJ, Chambers K, Rothchild K, Kjaergaard J, Papay R, Shu S. Kim JA, et al. Cancer Res. 2001 Mar 1;61(5):2031-7. Cancer Res. 2001. PMID: 11280763 - NK and CD8+ T cell-mediated eradication of poorly immunogenic B16-F10 melanoma by the combined action of IL-12 gene therapy and 4-1BB costimulation.
Xu D, Gu P, Pan PY, Li Q, Sato AI, Chen SH. Xu D, et al. Int J Cancer. 2004 Apr 20;109(4):499-506. doi: 10.1002/ijc.11696. Int J Cancer. 2004. PMID: 14991570 - Biochemical and immunological characteristics of 4-1BB (CD137) receptor and ligand and potential applications in cancer therapy.
Sica G, Chen L. Sica G, et al. Arch Immunol Ther Exp (Warsz). 1999;47(5):275-9. Arch Immunol Ther Exp (Warsz). 1999. PMID: 10604232 Review. - Therapeutic vaccination with tumor cells that engage CD137.
Hellstrom KE, Hellstrom I. Hellstrom KE, et al. J Mol Med (Berl). 2003 Feb;81(2):71-86. doi: 10.1007/s00109-002-0413-8. Epub 2003 Feb 8. J Mol Med (Berl). 2003. PMID: 12601523 Review.
Cited by
- Artificial antigen-presenting cells: the booster for the obtaining of functional adoptive cells.
Li J, Zhou W, Wang W. Li J, et al. Cell Mol Life Sci. 2024 Aug 31;81(1):378. doi: 10.1007/s00018-024-05412-y. Cell Mol Life Sci. 2024. PMID: 39215816 Free PMC article. Review. - Immunotherapy-induced cytotoxic T follicular helper cells reduce numbers of retrovirus-infected reservoir cells in B cell follicles.
Malyshkina A, Bayer W, Podschwadt P, Otto L, Karakoese Z, Sutter K, Bruderek K, Wang B, Lavender KJ, Santiago ML, Leipe PM, Elsner C, Esser S, Brandau S, Gunzer M, Dittmer U. Malyshkina A, et al. PLoS Pathog. 2023 Oct 26;19(10):e1011725. doi: 10.1371/journal.ppat.1011725. eCollection 2023 Oct. PLoS Pathog. 2023. PMID: 37883584 Free PMC article. - TCR-independent CD137 (4-1BB) signaling promotes CD8+-exhausted T cell proliferation and terminal differentiation.
Pichler AC, Carrié N, Cuisinier M, Ghazali S, Voisin A, Axisa PP, Tosolini M, Mazzotti C, Golec DP, Maheo S, do Souto L, Ekren R, Blanquart E, Lemaitre L, Feliu V, Joubert MV, Cannons JL, Guillerey C, Avet-Loiseau H, Watts TH, Salomon BL, Joffre O, Grinberg-Bleyer Y, Schwartzberg PL, Lucca LE, Martinet L. Pichler AC, et al. Immunity. 2023 Jul 11;56(7):1631-1648.e10. doi: 10.1016/j.immuni.2023.06.007. Epub 2023 Jun 30. Immunity. 2023. PMID: 37392737 Free PMC article. - Photoactivatable nanoagonists chemically programmed for pharmacokinetic tuning and in situ cancer vaccination.
Wan J, Ren L, Li X, He S, Fu Y, Xu P, Meng F, Xian S, Pu K, Wang H. Wan J, et al. Proc Natl Acad Sci U S A. 2023 Feb 21;120(8):e2210385120. doi: 10.1073/pnas.2210385120. Epub 2023 Feb 14. Proc Natl Acad Sci U S A. 2023. PMID: 36787350 Free PMC article. - Cowpea Mosaic Virus and Natural Killer Cell Agonism for In Situ Cancer Vaccination.
Koellhoffer EC, Steinmetz NF. Koellhoffer EC, et al. Nano Lett. 2022 Jul 13;22(13):5348-5356. doi: 10.1021/acs.nanolett.2c01328. Epub 2022 Jun 17. Nano Lett. 2022. PMID: 35713326 Free PMC article.
References
- Sica G, Chen L. Modulation of the immune response through 4-1BB. Adv Exp Med Biol. 2000;465:355–362. - PubMed
- Vinay DS, Kwon BS. Role of 4-1BB in immune responses. Semin Immunol. 1998;10:481–489. - PubMed
- Pollok KE, et al. Inducible T cell antigen 4-1BB. Analysis of expression and function. J Immunol. 1993;150:771–781. - PubMed
- DeBenedette MA, Shahinian A, Mak TW, Watts TH. Co-stimulation of CD28- T lymphocytes by 4-1BB ligand. J Immunol. 1997;158:551–559. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 CA085721/CA/NCI NIH HHS/United States
- T32 CA009127/CA/NCI NIH HHS/United States
- CA-09127/CA/NCI NIH HHS/United States
- CA-79915/CA/NCI NIH HHS/United States
- CA-85721/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous