Enumeration of the simian virus 40 early region elements necessary for human cell transformation - PubMed (original) (raw)
Enumeration of the simian virus 40 early region elements necessary for human cell transformation
William C Hahn et al. Mol Cell Biol. 2002 Apr.
Erratum in
- Mol Cell Biol 2002 May;22(10):3562
Abstract
While it is clear that cancer arises from the accumulation of genetic mutations that endow the malignant cell with the properties of uncontrolled growth and proliferation, the precise combinations of mutations that program human tumor cell growth remain unknown. The study of the transforming proteins derived from DNA tumor viruses in experimental models of transformation has provided fundamental insights into the process of cell transformation. We recently reported that coexpression of the simian virus 40 (SV40) early region (ER), the gene encoding the telomerase catalytic subunit (hTERT), and an oncogenic allele of the H-ras gene in normal human fibroblast, kidney epithelial, and mammary epithelial cells converted these cells to a tumorigenic state. Here we show that the SV40 ER contributes to tumorigenic transformation in the presence of hTERT and oncogenic H-ras by perturbing three intracellular pathways through the actions of the SV40 large T antigen (LT) and the SV40 small t antigen (ST). LT simultaneously disables the retinoblastoma (pRB) and p53 tumor suppressor pathways; however, complete transformation of human cells requires the additional perturbation of protein phosphatase 2A by ST. Expression of ST in this setting stimulates cell proliferation, permits anchorage-independent growth, and confers increased resistance to nutrient deprivation. Taken together, these observations define the elements of the SV40 ER required for the transformation of human cells and begin to delineate a set of intracellular pathways whose disruption, in aggregate, appears to be necessary to generate tumorigenic human cells.
Figures
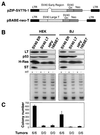
FIG. 1.
Tumorigenicity of cells expressing SV40 and HPV oncoproteins. (A) Schematic representation of the vector encoding the SV40 ER (pZIP-SV776-1) and the vector encoding only SV40 LT (pBABE-neo-T). The portion of pBABE-neo-T that encodes LT lacks the intron present in the SV40 ER. (B) Immunoblotting of cells expressing the SV40 ER or LT or HPV-16 E6 or E7. One hundred micrograms of total cell protein was separated on 7.5 to 15% gradient gels and immunoblotted with antibodies specific for LT (Pab101), p53 (Ab6), H-Ras (C20), and ST (Pab108 and Pab419). The level of LT expression is much greater than the level of ST expression. The LT exposure time was 1 min, while the ST exposure time was 30 min. TRAP assays were performed on 200 ng of total cellular protein. HT refers to heat-treated samples. An internal control band used to ensure PCR reactivity was present for each sample (data not shown). (C) AI growth and tumorigenicity of the indicated cell lines. To assess AI growth, 105 cells were plated in 0.4% Noble agar and colonies were counted 21 to 28 days after seeding. The mean and standard deviation of four experiments are shown. Tumor formation in immunodeficient mice was assessed by s.c. injection of 2 × 106 cells and is reported as the number of tumors identified/number of injection sites. LTR, long terminal repeat.
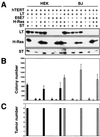
FIG. 2.
Analysis of cell lines expressing combinations of the LT, ST, hTERT, H-Ras, and HPV proteins. (A) HEK or BJ cells expressing the indicated combinations of introduced proteins were analyzed by immunoblotting of 100 μg of total cell protein and separation on 7.5 to 15% gradient gels. All of the cell lines expressed hTERT, as assessed by TRAP assay (data not shown). Cell lines created with the SV40 ER are depicted in the first column of HEK and BJ cells. As shown, although LT is expressed at a higher level than ST, LT and ST introduced from distinct retroviral constructs were expressed at levels similar to those in cells infected with a retrovirus that transduces the entire SV40 ER. The LT exposure time was 1 min, while the ST exposure time was 30 min. (B) AI colony formation. The mean and standard deviation of three experiments are shown. (C) Tumor formation in immunodeficient mice. The numbers of tumors observed after six s.c. injections are depicted. In panels B and C, HEK cells are represented by black bars and BJ fibroblasts are represented by dotted bars.
FIG. 3.
Effects of LT mutant forms on replicative senescence and crisis. (A) Schematic representation of LT mutant forms. LXCXE, binding site for pRB family members; NLS, nuclear localization signal; X, amino acid substitutions that disrupt LT function; WT, wild type. Δ434-444 has a small in-frame deletion that eliminates p53 binding. (B) Effects of LT mutant forms on replicative senescence in HEK cells. HEK cells were infected with a control retrovirus (open squares) or with a retrovirus encoding an LT mutant form. Wild-type LT (filled squares), K1 (filled triangles), H42Q (open circles), Δ434-444 (open triangles), N136 (filled diamonds), and 1137 (filled circles) are shown. (C) Effects of LT mutant forms on replicative senescence in BJ cells. Symbols are the same as in panel B. (D) Effects of LT mutant forms on crisis in HEK cells. HEK cells expressing LT with (squares) or without (diamonds) hTERT or the LT H42Q mutant form with (circles) or without (triangles) hTERT are shown. (E) Effects of LT mutant forms on crisis in BJ cells. hTERT was introduced after the indicated cells had bypassed replicative senescence. Symbols are the same as in panel D. (F) Effects of LT mutant forms on immortalization in BJ fibroblasts expressing hTERT prior to introduction of the mutant forms. Symbols are the same as in panel B. Open diamonds represent the proliferation of BJ fibroblasts infected with only a control retrovirus.
FIG. 4.
Effects of LT expression on resistance to _ras_-induced senescence. (A) Expression of H-Ras in BJ cells expressing the indicated LT mutant forms or a control vector and hTERT (data not shown). One hundred micrograms of total cell protein was analyzed. (B) Cell proliferation after introduction of H-Ras into BJ fibroblasts expressing a control vector (open diamonds), wild-type (WT) LT (filled squares), the K1 mutant form (filled triangles), the H42Q mutant form (open circles), the Δ434-444 mutant form (open triangles), the N136 mutant form (filled diamonds), and the 1137 mutant form (filled circles). (C) AI growth and tumorigenicity of the indicated cell lines. The mean and standard deviation of three experiments are shown. Tumor formation is reported as in Fig. 1.
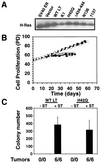
FIG. 5.
Perturbation of the pRB and p53 tumor suppressor pathways. (A) Expression of indicated proteins in BJ cells expressing LT, hTERT, and ras with (TERST) or without (TERV) ST or in BJ cells expressing a combination of cyclin D1 (D1), the R24C CDK4 mutant form, hTERT, and ras with (DRDTRST) or without (DRDTRV) ST. One hundred micrograms of total cell protein was separated on 7.5 to 15% gradient gels, transferred nitrocellulose, and sequentially immunoblotted. The p53DD mutant form is a truncated form of p53, stabilizes endogenous p53, and migrates at approximately 28 kDa (70). Relative molecular mass (kilodaltons [kd]) is indicated. The D1 and R24C CDK4 proteins are HA epitope tagged and migrate more slowly than the endogenous forms of these proteins. Both the HA-tagged and endogenous forms of these proteins are indicated by arrowheads. All of the cell lines express hTERT, as assessed by TRAP assay (data not shown). (B) Effects of cyclin D1 and R24C CDK4 expression on pRB phosphorylation. Immunoblotting demonstrating pRB phosphorylation of cells growing exponentially (subconfluent [S]) or at confluence (C). BJ cells expressing only hTERT (hTERT) were used as a control. (C) Effects of gamma irradiation (γ-irr). Parallel cultures of BJ fibroblasts expressing only hTERT (hTERT) or DRDTRST were treated with 5 Gy. The percentage of cells in each phase of the cell cycle was determined by BrdU incorporation and propidium iodide staining 24 h after irradiation. (D) AI growth and tumorigenicity of the indicated cell lines. The mean and standard deviation of six experiments are shown. Tumor formation is reported as in Fig. 1.
FIG. 6.
Mapping of the functional domain of ST required for transformation. (A) Schematic representation of ST mutant forms. Black bars represent the domain shared by LT and ST. The FLAG epitope tag is represented by the hatched region. WT, wild type. (B) Expression of ST mutant forms. One hundred micrograms of total cell protein was separated on 7.5 to 15% gradient gels and blotted with Pab108 and Pab419 or the M2 MAb specific for the FLAG epitope tag. Expression of the other introduced proteins was similar (data not shown). (C and D) AI growth and tumorigenicity of the indicated cell lines. The mean and standard deviation of three experiments are shown. Tumor formation is reported as in Fig. 1. Black bars represent HEK cells expressing LT, hTERT, and ras. Dotted bars represent BJ fibroblasts expressing LT, hTERT, and ras. Hatched bars represent BJ fibroblasts expressing the combination of cyclin D1, the R24C CDK4 mutant form, the p53DD mutant form, hTERT, and ras. kd, kilodaltons.
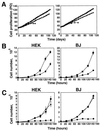
FIG. 7.
Effects of ST on cell proliferation. (A) ST does not affect immortalization. Proliferation of HEK or BJ cells expressing LT, hTERT, and ras with (squares) or without (circles) ST. For comparison, HEK or BJ cells expressing only LT are also depicted (diamonds). PD is defined as in Fig. 3. (B) Effects of ST on proliferation. HEK and BJ cells were grown in medium supplemented with the normal concentration of glutamine (3.75 mM). Symbols are as in panel A. (C) Effects of ST on cell proliferation at a limiting glutamine concentration (0.375 mM). Cells expressing LT, hTERT, and ras with (squares) or without (circles) ST and cells expressing LT, hTERT, and ST without ras (triangles) are shown. In panels B and C, the mean and standard deviation of three experiments are shown.
Similar articles
- Upregulation of miR-27a contributes to the malignant transformation of human bronchial epithelial cells induced by SV40 small T antigen.
Wang Q, Li DC, Li ZF, Liu CX, Xiao YM, Zhang B, Li XD, Zhao J, Chen LP, Xing XM, Tang SF, Lin YC, Lai YD, Yang P, Zeng JL, Xiao Q, Zeng XW, Lin ZN, Zhuang ZX, Zhuang SM, Chen W. Wang Q, et al. Oncogene. 2011 Sep 8;30(36):3875-86. doi: 10.1038/onc.2011.103. Epub 2011 Apr 4. Oncogene. 2011. PMID: 21460851 - Inhibition of SV40 large T antigen induced apoptosis by small T antigen.
Kolzau T, Hansen RS, Zahra D, Reddel RR, Braithwaite AW. Kolzau T, et al. Oncogene. 1999 Sep 30;18(40):5598-603. doi: 10.1038/sj.onc.1202942. Oncogene. 1999. PMID: 10523837 - The minimal set of genetic alterations required for conversion of primary human fibroblasts to cancer cells in the subrenal capsule assay.
Sun B, Chen M, Hawks CL, Pereira-Smith OM, Hornsby PJ. Sun B, et al. Neoplasia. 2005 Jun;7(6):585-93. doi: 10.1593/neo.05172. Neoplasia. 2005. PMID: 16036109 Free PMC article. - SV40 early region oncoproteins and human cell transformation.
Chen W, Hahn WC. Chen W, et al. Histol Histopathol. 2003 Apr;18(2):541-50. doi: 10.14670/HH-18.541. Histol Histopathol. 2003. PMID: 12647805 Review. - Immortalized normal human lung epithelial cell models for studying lung cancer biology.
Sato M, Shay JW, Minna JD. Sato M, et al. Respir Investig. 2020 Sep;58(5):344-354. doi: 10.1016/j.resinv.2020.04.005. Epub 2020 Jun 22. Respir Investig. 2020. PMID: 32586780 Review.
Cited by
- HDAC inhibitors induce tumor-cell-selective pro-apoptotic transcriptional responses.
Bolden JE, Shi W, Jankowski K, Kan CY, Cluse L, Martin BP, MacKenzie KL, Smyth GK, Johnstone RW. Bolden JE, et al. Cell Death Dis. 2013 Feb 28;4(2):e519. doi: 10.1038/cddis.2013.9. Cell Death Dis. 2013. PMID: 23449455 Free PMC article. - Structural basis of PP2A inhibition by small t antigen.
Cho US, Morrone S, Sablina AA, Arroyo JD, Hahn WC, Xu W. Cho US, et al. PLoS Biol. 2007 Aug;5(8):e202. doi: 10.1371/journal.pbio.0050202. PLoS Biol. 2007. PMID: 17608567 Free PMC article. - A mitochondrial ROS pathway controls matrix metalloproteinase 9 levels and invasive properties in RAS-activated cancer cells.
Mori K, Uchida T, Yoshie T, Mizote Y, Ishikawa F, Katsuyama M, Shibanuma M. Mori K, et al. FEBS J. 2019 Feb;286(3):459-478. doi: 10.1111/febs.14671. Epub 2018 Oct 13. FEBS J. 2019. PMID: 30281903 Free PMC article. - Human polyomaviruses and cancer: an overview.
Prado JCM, Monezi TA, Amorim AT, Lino V, Paladino A, Boccardo E. Prado JCM, et al. Clinics (Sao Paulo). 2018 Oct 11;73(suppl 1):e558s. doi: 10.6061/clinics/2018/e558s. Clinics (Sao Paulo). 2018. PMID: 30328951 Free PMC article. Review. - NKAP Must Associate with HDAC3 to Regulate Hematopoietic Stem Cell Maintenance and Survival.
Shapiro MJ, Lehrke MJ, Chung JY, Romero Arocha S, Shapiro VS. Shapiro MJ, et al. J Immunol. 2019 Apr 15;202(8):2287-2295. doi: 10.4049/jimmunol.1800862. Epub 2019 Feb 25. J Immunol. 2019. PMID: 30804042 Free PMC article.
References
- Ali, S. H., and J. A. DeCaprio. 2001. Cellular transformation by SV40 large T antigen: interaction with host proteins. Semin. Cancer Biol. 11:15-23. - PubMed
- Bikel, I., X. Montano, M. E. Agha, M. Brown, M. McCormack, J. Boltax, and D. M. Livingston. 1987. SV40 small t antigen enhances the transformation activity of limiting concentrations of SV40 large T antigen. Cell 48:321-330. - PubMed
- Bishop, J. M., and R. A. Weinberg (ed.). 1996. Molecular oncology. Scientific American, Inc., New York. N.Y.
- Bocchetta, M., I. Di Resta, A. Powers, R. Fresco, A. Tosolini, J. R. Testa, H. I. Pass, P. Rizzo, and M. Carbone. 2000. Human mesothelial cells are unusually susceptible to simian virus 40-mediated transformation and asbestos cocarcinogenicity. Proc. Natl. Acad. Sci. USA 97:10214-10219. - PMC - PubMed
- Bodnar, A. G., M. Ouellette, M. Frolkis, S. E. Holt, C. P. Chiu, G. B. Morin, C. B. Harley, J. W. Shay, S. Lichtsteiner, and W. E. Wright. 1998. Extension of life-span by introduction of telomerase into normal human cells. Science 279:349-352. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- K08 HL004463/HL/NHLBI NIH HHS/United States
- K01 CA094223/CA/NCI NIH HHS/United States
- P01 CA50661/CA/NCI NIH HHS/United States
- R01 CA78461/CA/NCI NIH HHS/United States
- R01 CA63113/CA/NCI NIH HHS/United States
- K08 HL04463/HL/NHLBI NIH HHS/United States
- R01 CA078461/CA/NCI NIH HHS/United States
- P01 CA050661/CA/NCI NIH HHS/United States
- R01 CA063113/CA/NCI NIH HHS/United States
- K01 CA94223/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous