Intracellular retention of glycosylphosphatidyl inositol-linked proteins in caveolin-deficient cells - PubMed (original) (raw)
Intracellular retention of glycosylphosphatidyl inositol-linked proteins in caveolin-deficient cells
Federica Sotgia et al. Mol Cell Biol. 2002 Jun.
Abstract
The relationship between glycosylphosphatidyl inositol (GPI)-linked proteins and caveolins remains controversial. Here, we derived fibroblasts from Cav-1 null mouse embryos to study the behavior of GPI-linked proteins in the absence of caveolins. These cells lack morphological caveolae, do not express caveolin-1, and show a approximately 95% down-regulation in caveolin-2 expression; these cells also do not express caveolin-3, a muscle-specific caveolin family member. As such, these caveolin-deficient cells represent an ideal tool to study the role of caveolins in GPI-linked protein sorting. We show that in Cav-1 null cells GPI-linked proteins are preferentially retained in an intracellular compartment that we identify as the Golgi complex. This intracellular pool of GPI-linked proteins is not degraded and remains associated with intracellular lipid rafts as judged by its Triton insolubility. In contrast, GPI-linked proteins are transported to the plasma membrane in wild-type cells, as expected. Furthermore, recombinant expression of caveolin-1 or caveolin-3, but not caveolin-2, in Cav-1 null cells complements this phenotype and restores the cell surface expression of GPI-linked proteins. This is perhaps surprising, as GPI-linked proteins are confined to the exoplasmic leaflet of the membrane, while caveolins are cytoplasmically oriented membrane proteins. As caveolin-1 normally undergoes palmitoylation on three cysteine residues (133, 143, and 156), we speculated that palmitoylation might mechanistically couple caveolin-1 to GPI-linked proteins. In support of this hypothesis, we show that palmitoylation of caveolin-1 on residues 143 and 156, but not residue 133, is required to restore cell surface expression of GPI-linked proteins in this complementation assay. We also show that another lipid raft-associated protein, c-Src, is retained intracellularly in Cav-1 null cells. Thus, Golgi-associated caveolins and caveola-like vesicles could represent part of the transport machinery that is necessary for efficiently moving lipid rafts and their associated proteins from the trans-Golgi to the plasma membrane. In further support of these findings, GPI-linked proteins were also retained intracellularly in tissue samples derived from Cav-1 null mice (i.e., lung endothelial and renal epithelial cells) and Cav-3 null mice (skeletal muscle fibers).
Figures
FIG. 1.
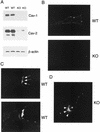
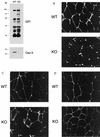
Phenotypic behavior of GPI-anchored proteins in lung tissue from Cav-1 null mice. (A) GPI Western blot analysis. The expression levels of lung GPI-anchored proteins remain unchanged in Cav-1 null mice compared with those of wild-type control mice. Lung tissue samples from wild-type and Cav-1 null mice were homogenized in lysis buffer, and 20 μg of lysate from each sample was separated by SDS-PAGE. After transfer to nitrocellulose, the blots were subsequently subjected to proaerolysin overlay to visualize GPI-linked proteins or to caveolin-1 immunoblotting. (B and C) Cellular fractionation. GPI-anchored proteins were targeted to lipid rafts/caveola-enriched fractions in lung tissue from wild-type and Cav-1 null mice. Lipid rafts/caveola microdomains were separated from other cellular constituents by using sucrose flotation gradients (see Materials and Methods). Lung tissue from wild-type and Cav-1 null mice was homogenized thoroughly in lysis buffer containing 1% Triton X-100 and subjected to sucrose gradient centrifugation. Twelve 1-ml fractions were collected, and 10 μg of each fraction was separated by SDS-PAGE and transferred to nitrocellulose. The distribution of GPI-anchored proteins was analyzed by proaerolysin overlay. Note that GPI-anchored proteins cofractionate with caveolin-1 (fractions 4 and 5) in lung tissue from wild-type mice (B). However, GPI-anchored proteins are still targeted to lipid rafts (fractions 4 and 5) in lung tissue from Cav-1 null mice (C). (D) CA-IV immunostaining. GPI-linked proteins show differences in their localization patterns in lung tissue from Cav-1 null mice. Lung paraffin sections from wild-type and Cav-1 null mice were labeled with an antibody directed against an abundant lung endothelial GPI-anchored protein, CA-IV. Bound primary antibodies were detected with a fluorescently labeled secondary antibody. Nuclear counterstaining was performed using DAPI. Top, CA-IV localizes at the plasma membrane in wild-type lung endothelial cells (see arrows). Bottom, GPI-anchored CA-IV shows a perinuclear localization pattern in Cav-1 null lung endothelial cells (see arrowhead); arrows point to the cell surface.
FIG. 1.
Phenotypic behavior of GPI-anchored proteins in lung tissue from Cav-1 null mice. (A) GPI Western blot analysis. The expression levels of lung GPI-anchored proteins remain unchanged in Cav-1 null mice compared with those of wild-type control mice. Lung tissue samples from wild-type and Cav-1 null mice were homogenized in lysis buffer, and 20 μg of lysate from each sample was separated by SDS-PAGE. After transfer to nitrocellulose, the blots were subsequently subjected to proaerolysin overlay to visualize GPI-linked proteins or to caveolin-1 immunoblotting. (B and C) Cellular fractionation. GPI-anchored proteins were targeted to lipid rafts/caveola-enriched fractions in lung tissue from wild-type and Cav-1 null mice. Lipid rafts/caveola microdomains were separated from other cellular constituents by using sucrose flotation gradients (see Materials and Methods). Lung tissue from wild-type and Cav-1 null mice was homogenized thoroughly in lysis buffer containing 1% Triton X-100 and subjected to sucrose gradient centrifugation. Twelve 1-ml fractions were collected, and 10 μg of each fraction was separated by SDS-PAGE and transferred to nitrocellulose. The distribution of GPI-anchored proteins was analyzed by proaerolysin overlay. Note that GPI-anchored proteins cofractionate with caveolin-1 (fractions 4 and 5) in lung tissue from wild-type mice (B). However, GPI-anchored proteins are still targeted to lipid rafts (fractions 4 and 5) in lung tissue from Cav-1 null mice (C). (D) CA-IV immunostaining. GPI-linked proteins show differences in their localization patterns in lung tissue from Cav-1 null mice. Lung paraffin sections from wild-type and Cav-1 null mice were labeled with an antibody directed against an abundant lung endothelial GPI-anchored protein, CA-IV. Bound primary antibodies were detected with a fluorescently labeled secondary antibody. Nuclear counterstaining was performed using DAPI. Top, CA-IV localizes at the plasma membrane in wild-type lung endothelial cells (see arrows). Bottom, GPI-anchored CA-IV shows a perinuclear localization pattern in Cav-1 null lung endothelial cells (see arrowhead); arrows point to the cell surface.
FIG. 1.
Phenotypic behavior of GPI-anchored proteins in lung tissue from Cav-1 null mice. (A) GPI Western blot analysis. The expression levels of lung GPI-anchored proteins remain unchanged in Cav-1 null mice compared with those of wild-type control mice. Lung tissue samples from wild-type and Cav-1 null mice were homogenized in lysis buffer, and 20 μg of lysate from each sample was separated by SDS-PAGE. After transfer to nitrocellulose, the blots were subsequently subjected to proaerolysin overlay to visualize GPI-linked proteins or to caveolin-1 immunoblotting. (B and C) Cellular fractionation. GPI-anchored proteins were targeted to lipid rafts/caveola-enriched fractions in lung tissue from wild-type and Cav-1 null mice. Lipid rafts/caveola microdomains were separated from other cellular constituents by using sucrose flotation gradients (see Materials and Methods). Lung tissue from wild-type and Cav-1 null mice was homogenized thoroughly in lysis buffer containing 1% Triton X-100 and subjected to sucrose gradient centrifugation. Twelve 1-ml fractions were collected, and 10 μg of each fraction was separated by SDS-PAGE and transferred to nitrocellulose. The distribution of GPI-anchored proteins was analyzed by proaerolysin overlay. Note that GPI-anchored proteins cofractionate with caveolin-1 (fractions 4 and 5) in lung tissue from wild-type mice (B). However, GPI-anchored proteins are still targeted to lipid rafts (fractions 4 and 5) in lung tissue from Cav-1 null mice (C). (D) CA-IV immunostaining. GPI-linked proteins show differences in their localization patterns in lung tissue from Cav-1 null mice. Lung paraffin sections from wild-type and Cav-1 null mice were labeled with an antibody directed against an abundant lung endothelial GPI-anchored protein, CA-IV. Bound primary antibodies were detected with a fluorescently labeled secondary antibody. Nuclear counterstaining was performed using DAPI. Top, CA-IV localizes at the plasma membrane in wild-type lung endothelial cells (see arrows). Bottom, GPI-anchored CA-IV shows a perinuclear localization pattern in Cav-1 null lung endothelial cells (see arrowhead); arrows point to the cell surface.
FIG. 1.
Phenotypic behavior of GPI-anchored proteins in lung tissue from Cav-1 null mice. (A) GPI Western blot analysis. The expression levels of lung GPI-anchored proteins remain unchanged in Cav-1 null mice compared with those of wild-type control mice. Lung tissue samples from wild-type and Cav-1 null mice were homogenized in lysis buffer, and 20 μg of lysate from each sample was separated by SDS-PAGE. After transfer to nitrocellulose, the blots were subsequently subjected to proaerolysin overlay to visualize GPI-linked proteins or to caveolin-1 immunoblotting. (B and C) Cellular fractionation. GPI-anchored proteins were targeted to lipid rafts/caveola-enriched fractions in lung tissue from wild-type and Cav-1 null mice. Lipid rafts/caveola microdomains were separated from other cellular constituents by using sucrose flotation gradients (see Materials and Methods). Lung tissue from wild-type and Cav-1 null mice was homogenized thoroughly in lysis buffer containing 1% Triton X-100 and subjected to sucrose gradient centrifugation. Twelve 1-ml fractions were collected, and 10 μg of each fraction was separated by SDS-PAGE and transferred to nitrocellulose. The distribution of GPI-anchored proteins was analyzed by proaerolysin overlay. Note that GPI-anchored proteins cofractionate with caveolin-1 (fractions 4 and 5) in lung tissue from wild-type mice (B). However, GPI-anchored proteins are still targeted to lipid rafts (fractions 4 and 5) in lung tissue from Cav-1 null mice (C). (D) CA-IV immunostaining. GPI-linked proteins show differences in their localization patterns in lung tissue from Cav-1 null mice. Lung paraffin sections from wild-type and Cav-1 null mice were labeled with an antibody directed against an abundant lung endothelial GPI-anchored protein, CA-IV. Bound primary antibodies were detected with a fluorescently labeled secondary antibody. Nuclear counterstaining was performed using DAPI. Top, CA-IV localizes at the plasma membrane in wild-type lung endothelial cells (see arrows). Bottom, GPI-anchored CA-IV shows a perinuclear localization pattern in Cav-1 null lung endothelial cells (see arrowhead); arrows point to the cell surface.
FIG. 2.
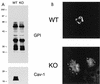
Intracellular retention of GPI-linked proteins in Cav-1-deficient 3T3 MEFs. (A) Caveolin Western blot analysis. 3T3 MEFs from Cav-1 knockout (KO) mice show an absence of caveolin-1 and severely reduced caveolin-2 levels. Samples containing 10 μg of lysates from wild-type (WT) and Cav-1 null MEFs were loaded in each lane, subjected to SDS-PAGE, and immunoblotted with an anti-Cav-1 MAb (clone 2297) or with an anti-Cav-2 MAb (clone 26). Equal protein loading was assessed using an anti-β-actin MAb. Results for two independent clones of each genotype are shown. (B) GPI cell surface labeling. Wild-type and Cav-1 null MEFs were grown on coverslips at a density of ∼70 to 80% confluency. After fixation, the cells were not permeabilized. Instead, they were directly labeled with 10−8 M proaerolysin and then incubated with an anti-aerolysin MAb. Bound primary antibodies were visualized with an FITC-conjugated anti-mouse antibody (see Materials and Methods). Note that in wild-type MEFs, GPI-anchored proteins show a punctate pattern of cell surface staining, as expected. In striking contrast, Cav-1 null MEFs show little or no detectable cell surface labeling of GPI-linked proteins. (C and D) GPI staining after detergent permeabilization. Wild-type and Cav-1 null MEFs were grown on coverslips at a density of ∼70 to 80% confluency. After fixation, the cells were permeabilized and then labeled with 10−8 M proaerolysin. Bound proaerolysin was visualized as described above in the legend for panel B. (C) In wild-type MEFs, GPI-anchored proteins were targeted to the plasma membrane and often assumed a polarized distribution. Arrows point at the cell surface. A longer exposure is also shown in the lower panel to illustrate the overall shape and contour of the cells. (D) In Cav-1 null MEFs, GPI-anchored proteins were primarily retained intracellularly in a perinuclear compartment (arrowheads). N, nucleus.
FIG.3.
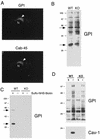
GPI-linked proteins are preferentially retained within lipid rafts at the level of the Golgi complex in Cav-1 null MEFs. (A) Double labeling with a Golgi marker protein. Formaldehyde-fixed and permeabilized Cav-1 null MEFs were double labeled with proaerolysin to visualize GPI-linked proteins and with Cab45, an endogenous Golgi marker protein. Bound primary antibodies were visualized with distinctly tagged secondary antibodies (see Materials and Methods). Note that the distributions of GPI-anchored proteins and Cab-45 precisely coincide, identifying the perinuclear region as the Golgi complex (arrowhead). N, nucleus. (B) GPI Western blot analysis. The expression levels of GPI-anchored proteins remained unchanged in Cav-1 null MEFs, compared with wild-type (WT) control MEFs. A 10-μg sample of cell lysate was loaded in each lane and separated by SDS-PAGE. The blots were subsequently subjected to proaerolysin overlay analysis to visualize GPI-linked proteins. Note the two major GPI-linked proteins with molecular masses of ∼35 and ∼60 kDa (see arrows). KO, knockout. (C) Biochemical detection of cell surface GPI-linked proteins. To detect only cell surface GPI-anchored proteins, we used cell surface biotinylation and concentrated the biotinylated GPI-linked proteins by phase separation using the detergent Triton X-114 and precipitation with streptavidin agarose beads (see Materials and Methods). The recovered biotinylated GPI-linked proteins were then visualized via the proaerolysin overlay assay. Note that by using this approach, we successfully detected the two major endogenous GPI-linked proteins (∼35 and ∼60 kDa; see arrows) at the cell surface in wild-type MEFs. Importantly, visualization of these GPI-linked proteins was strictly dependent on cell surface biotinylation, as they were not observed if cell surface biotinylation was omitted. In striking contrast, no cell surface GPI-linked proteins were detected in Cav-1 null MEFs. +, samples subjected to cell surface biotinylation with sulfo-NHS-biotin; −, the biotinylation step was omitted (a critical negative control). (D) GPI detergent insolubility. GPI-anchored proteins are detergent insoluble both in wild-type and knockout MEFs. After incubation of wild-type and Cav-1 null MEFs with a buffer containing 1% Triton X-100, the soluble fraction was collected. Then, the insoluble fraction was extracted using 1% SDS. Equal volumes of the soluble fraction and insoluble fraction were resolved by SDS-PAGE (10% acrylamide) and analyzed by proaerolysin overlay or caveolin-1 immunoblotting. Note that a caveolin-1 deficiency did not affect the detergent solubility of the GPI-anchored proteins; they remain predominantly Triton insoluble, indicative of their association with lipid rafts.
FIG. 4.
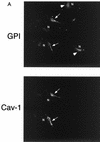
Recombinant expression of caveolin-1 or caveolin-3 restores the robust cell surface expression of GPI-linked proteins in Cav-1 null MEFs. Cav-1 null MEFs were transiently transfected with full-length cDNAs encoding either caveolin-1, caveolin-2, or caveolin-3. Thirty-six hours posttransfection, cells were formaldehyde fixed and doubly immunostained with proaerolysin and with either anti-Cav-1, anti-Cav-2 or anti-Cav-3 PAb. (A) Caveolin-1. Recombinant expression of caveolin-1 rescues the ability of GPI-anchored proteins to reach the plasma membrane. Note that both GPI-linked proteins and caveolin-1 colocalized to the plasma membrane in caveolin-1-transfected cells (arrows). In the same field, two untransfected cells that did not express caveolin-1 showed the retention of GPI-anchored proteins in the Golgi complex (arrowheads). N, nucleus. (B) Caveolin-2. Recombinant expression of caveolin-2 failed to restore the cell surface expression of GPI-linked proteins. Note that both GPI-anchored proteins and caveolin-2 were colocalized to the Golgi complex (arrowheads). The image shown is that of a caveolin-2-transfected cell. N, nucleus. (C) Caveolin-3. Recombinant expression of caveolin-3 rescued the ability of GPI-anchored proteins to reach the plasma membrane. Note that both GPI-linked proteins and caveolin-3 colocalized to the plasma membrane in the caveolin-3-transfected cell (arrows). In the same field, an untransfected cell that did not express caveolin-3 showed the retention of GPI-anchored proteins in the Golgi complex (arrowhead). N, nucleus.
FIG. 4.
Recombinant expression of caveolin-1 or caveolin-3 restores the robust cell surface expression of GPI-linked proteins in Cav-1 null MEFs. Cav-1 null MEFs were transiently transfected with full-length cDNAs encoding either caveolin-1, caveolin-2, or caveolin-3. Thirty-six hours posttransfection, cells were formaldehyde fixed and doubly immunostained with proaerolysin and with either anti-Cav-1, anti-Cav-2 or anti-Cav-3 PAb. (A) Caveolin-1. Recombinant expression of caveolin-1 rescues the ability of GPI-anchored proteins to reach the plasma membrane. Note that both GPI-linked proteins and caveolin-1 colocalized to the plasma membrane in caveolin-1-transfected cells (arrows). In the same field, two untransfected cells that did not express caveolin-1 showed the retention of GPI-anchored proteins in the Golgi complex (arrowheads). N, nucleus. (B) Caveolin-2. Recombinant expression of caveolin-2 failed to restore the cell surface expression of GPI-linked proteins. Note that both GPI-anchored proteins and caveolin-2 were colocalized to the Golgi complex (arrowheads). The image shown is that of a caveolin-2-transfected cell. N, nucleus. (C) Caveolin-3. Recombinant expression of caveolin-3 rescued the ability of GPI-anchored proteins to reach the plasma membrane. Note that both GPI-linked proteins and caveolin-3 colocalized to the plasma membrane in the caveolin-3-transfected cell (arrows). In the same field, an untransfected cell that did not express caveolin-3 showed the retention of GPI-anchored proteins in the Golgi complex (arrowhead). N, nucleus.
FIG. 4.
Recombinant expression of caveolin-1 or caveolin-3 restores the robust cell surface expression of GPI-linked proteins in Cav-1 null MEFs. Cav-1 null MEFs were transiently transfected with full-length cDNAs encoding either caveolin-1, caveolin-2, or caveolin-3. Thirty-six hours posttransfection, cells were formaldehyde fixed and doubly immunostained with proaerolysin and with either anti-Cav-1, anti-Cav-2 or anti-Cav-3 PAb. (A) Caveolin-1. Recombinant expression of caveolin-1 rescues the ability of GPI-anchored proteins to reach the plasma membrane. Note that both GPI-linked proteins and caveolin-1 colocalized to the plasma membrane in caveolin-1-transfected cells (arrows). In the same field, two untransfected cells that did not express caveolin-1 showed the retention of GPI-anchored proteins in the Golgi complex (arrowheads). N, nucleus. (B) Caveolin-2. Recombinant expression of caveolin-2 failed to restore the cell surface expression of GPI-linked proteins. Note that both GPI-anchored proteins and caveolin-2 were colocalized to the Golgi complex (arrowheads). The image shown is that of a caveolin-2-transfected cell. N, nucleus. (C) Caveolin-3. Recombinant expression of caveolin-3 rescued the ability of GPI-anchored proteins to reach the plasma membrane. Note that both GPI-linked proteins and caveolin-3 colocalized to the plasma membrane in the caveolin-3-transfected cell (arrows). In the same field, an untransfected cell that did not express caveolin-3 showed the retention of GPI-anchored proteins in the Golgi complex (arrowhead). N, nucleus.
FIG.5.
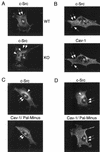
Expression of palmitoylated caveolin-1 is required for efficient transport of GPI-linked proteins to the plasma membrane in Cav-1 null MEFs. (A) Schematic diagram. GPI-linked proteins are confined to the exoplasmic leaflet of the membrane, while caveolins are cytoplasmically oriented membrane proteins. As caveolin-1 normally undergoes palmitoylation on three cysteine residues (133, 143, and 156), we speculate that caveolin-1 palmitoylation might mechanistically serve to couple caveolin-1 to GPI-linked proteins. In direct support of this hypothesis, in vivo chemical cross-linking studies with an iodinated derivative of GM1 (a glycosphingolipid) showed that GM1 interacted directly with caveolin-1 in intact cells, despite the fact that GM1 (exoplasmic) and caveolin-1 (cytoplasmic) are on opposite sides of the lipid bilayer (25). The acyl group-dependent association of caveolin-1 with cholesterol (94) may also link the cytoplasmic and exoplasmic leaflets of the lipid bilayer, as cholesterol is present in both leaflets and can potentially form dimers that span the membrane-acting as a bridge (33). Alternatively, palmitoylation of caveolin-1 may simply affect the global organization of lipid rafts, thereby indirectly facilitating the recruitment of GPI-linked proteins. (B) Palmitoylation-deficient caveolin-1 mutants. A representation of the N-terminal, membrane-spanning, and C-terminal domains of caveolin-1 is shown. Each cysteine residue within caveolin-1 that is normally palmitoylated was mutated to a serine, either individually or in combination. (C to F) GPI staining after detergent permeabilization. Cav-1 null MEFs were transfected with each of the full-length cDNAs encoding the above indicated caveolin-1 palmitoylation mutants. Thirty-six hours posttransfection, cells were formaldehyde fixed and doubly immunostained with proaerolysin and with an anti-caveolin-1 PAb (N20). Note that palmitoylation-deficient caveolin-1 (C133, 143, and 156S-Pal Minus) completely failed to rescue the cell surface transport of GPI-linked proteins. However, recombinant expression of caveolin-1 (C133S) was sufficient to recruit GPI-anchored proteins to the plasma membrane, although with a lower efficiency than wild-type caveolin-1. In contrast, recombinant expression of caveolin-1 (C143S) and caveolin-1 (C156S) failed to rescue the cell surface expression of GPI-linked proteins. Thus, palmitoylated caveolin-1 normally functions as a molecular escort to allow the efficient cell surface transport of GPI-linked proteins. (C to F) Arrows point at the cell surface, while arrowheads point at the Golgi complex. N, nucleus.
FIG.5.
Expression of palmitoylated caveolin-1 is required for efficient transport of GPI-linked proteins to the plasma membrane in Cav-1 null MEFs. (A) Schematic diagram. GPI-linked proteins are confined to the exoplasmic leaflet of the membrane, while caveolins are cytoplasmically oriented membrane proteins. As caveolin-1 normally undergoes palmitoylation on three cysteine residues (133, 143, and 156), we speculate that caveolin-1 palmitoylation might mechanistically serve to couple caveolin-1 to GPI-linked proteins. In direct support of this hypothesis, in vivo chemical cross-linking studies with an iodinated derivative of GM1 (a glycosphingolipid) showed that GM1 interacted directly with caveolin-1 in intact cells, despite the fact that GM1 (exoplasmic) and caveolin-1 (cytoplasmic) are on opposite sides of the lipid bilayer (25). The acyl group-dependent association of caveolin-1 with cholesterol (94) may also link the cytoplasmic and exoplasmic leaflets of the lipid bilayer, as cholesterol is present in both leaflets and can potentially form dimers that span the membrane-acting as a bridge (33). Alternatively, palmitoylation of caveolin-1 may simply affect the global organization of lipid rafts, thereby indirectly facilitating the recruitment of GPI-linked proteins. (B) Palmitoylation-deficient caveolin-1 mutants. A representation of the N-terminal, membrane-spanning, and C-terminal domains of caveolin-1 is shown. Each cysteine residue within caveolin-1 that is normally palmitoylated was mutated to a serine, either individually or in combination. (C to F) GPI staining after detergent permeabilization. Cav-1 null MEFs were transfected with each of the full-length cDNAs encoding the above indicated caveolin-1 palmitoylation mutants. Thirty-six hours posttransfection, cells were formaldehyde fixed and doubly immunostained with proaerolysin and with an anti-caveolin-1 PAb (N20). Note that palmitoylation-deficient caveolin-1 (C133, 143, and 156S-Pal Minus) completely failed to rescue the cell surface transport of GPI-linked proteins. However, recombinant expression of caveolin-1 (C133S) was sufficient to recruit GPI-anchored proteins to the plasma membrane, although with a lower efficiency than wild-type caveolin-1. In contrast, recombinant expression of caveolin-1 (C143S) and caveolin-1 (C156S) failed to rescue the cell surface expression of GPI-linked proteins. Thus, palmitoylated caveolin-1 normally functions as a molecular escort to allow the efficient cell surface transport of GPI-linked proteins. (C to F) Arrows point at the cell surface, while arrowheads point at the Golgi complex. N, nucleus.
FIG.6.
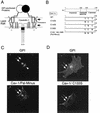
In Cav-3 null mouse skeletal muscle fibers, GPI-anchored proteins are expressed at normal levels but display an abnormal localization pattern and are retained intracellularly. (A) GPI Western blot analysis. Protein lysates were prepared from skeletal muscle biopsies taken from wild-type (WT) and Cav-3 null (KO) mice. After SDS-PAGE and transfer to nitrocellulose, the blots were subjected to proaerolysin overlay to detect GPI-linked proteins or to caveolin-3 immunoblotting. Note that the GPI-anchored proteins were expressed at normal levels in the skeletal muscle of Cav-3 null mice, compared to those of wild-type control mice. (B to D) Immunohistochemistry. Skeletal muscle tissue frozen sections were prepared from wild-type and Cav-3 null mice. These frozen sections were then immunostained with (i) proaerolysin to reveal the distribution of GPI-linked proteins (B), (ii) a PAb directed against T-cadherin, an endogenous GPI-linked protein that is prominently expressed in skeletal muscle (C), or (iii) a MAb directed against β-dystroglycan (D). Note the intracellular retention of GPI-linked proteins (B and C) in Cav-3 null skeletal muscle fibers; in contrast, the neighboring endothelial cells (arrows) which express caveolin-1 showed a normal labeling pattern.
FIG. 7.
Intracellular retention of GPI-linked proteins in the renal tubules of Cav-1 null mice. (A) GPI Western blot analysis. Protein lysates were prepared from kidneys harvested from wild-type (WT) and Cav-1 null (KO) mice. After SDS-PAGE and transfer to nitrocellulose, the blots were subjected to proaerolysin overlay to detect GPI-linked proteins or to caveolin-1 immunoblotting. Note that the GPI-anchored proteins were expressed at normal levels in the kidneys of Cav-1 null mice, compared to those of wild-type control mice. (B) Tamm-Horsfall immunostaining. Kidney paraffin sections prepared from wild-type and Cav-1 null mice were immunostained with a polyclonal sheep antibody directed against the Tamm-Horsfall protein, a GPI-anchored protein endogenously expressed in the renal tubules. Note that in wild-type mice, the Tamm-Horsfall protein was properly localized at the apical cell surface of renal tubular epithelial cells and showed a ring-like staining pattern, as expected. In contrast, in Cav-1 null mice, the Tamm-Horsfall protein was largely excluded from the apical plasma membrane and was found mainly intracellularly in a vesicular perinuclear compartment that was proximal to the apical cell surface. N, nucleus.
FIG.8.
Perinuclear retention of c-Src in Cav-1-deficient MEFs: recombinant expression of caveolin-1 restores the normal plasma membrane distribution of c-Src. (A) c-Src immunostaining. Wild-type (WT) and Cav-1 null (KO) MEFs were transiently transfected with the cDNA encoding c-Src. Cells were then fixed, permeabilized, and immunolabeled with a specific antibody directed against c-Src. Note that in wild-type MEFs, c-Src resided at the plasma membrane, as expected. In contrast, in Cav-1 null MEFs, c-Src was preferentially retained at an intracellular perinuclear site. (B) Wild-type caveolin-1. Cav-1 null MEFs were cotransfected with the cDNAs encoding c-Src and wild-type caveolin-1. c-Src expression was detected with a specific mouse MAb; caveolin-1 expression was detected with a rabbit PAb (N-20). Bound primary antibodies were visualized by incubation with distinctly tagged fluorescent secondary antibodies. Cells expressing both transfected gene products were selected for imaging. Note that expression of wild-type caveolin-1 restored the normal plasma membrane distribution of c-Src. (C and D) Palmitoylation-deficient caveolin-1. Cav-1 null MEFs were cotransfected with the cDNAs encoding c-Src and palmitoylation-deficient (C133, 143, and 156S-Pal Minus) caveolin-1. The recombinant expression of c-Src and caveolin-1 was detected as described above for panel B. Note that palmitoylation-deficient caveolin-1 showed little (C) or no (D) ability to rescue the plasma membrane targeting of c-Src. Thus, we conclude that palmitoylation of caveolin-1 is normally required for the efficient cell surface transport of lipid rafts that contain GPI-linked proteins and Src-family kinases. (A to C) Arrows point at the cell surface, while arrowheads point at the Golgi complex. N, nucleus.
FIG. 9.
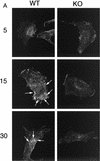
Clustering and internalization of CT-B is impaired in Cav-1 null MEFs. Wild-type and Cav-1 null 3T3 MEFs were incubated with CT-B for 30 min on ice, unbound material was removed, and the cells were then warmed to 37°C to allow clustering and internalization to occur. CT-B binding and internalization were visualized by immunostaining with anti-CT IgG. (A) MEFs after 5, 15, and 30 min of internalization. Note that the cell surface clustering and internalization of CT-B was clearly impaired in Cav-1 null (KO) MEFs. Arrows point at areas of cell surface clustering and internalization in wild-type (WT) MEFs. (B) MEFs after 45 min of internalization. Note that little or no cell surface clustering and internalization was apparent in Cav-1 null MEFs. In contrast, by 45 min, internalization of CT-B in wild-type MEFs was virtually complete, with a high concentration of CT-B accumulating in a perinuclear compartment (arrows).
FIG. 9.
Clustering and internalization of CT-B is impaired in Cav-1 null MEFs. Wild-type and Cav-1 null 3T3 MEFs were incubated with CT-B for 30 min on ice, unbound material was removed, and the cells were then warmed to 37°C to allow clustering and internalization to occur. CT-B binding and internalization were visualized by immunostaining with anti-CT IgG. (A) MEFs after 5, 15, and 30 min of internalization. Note that the cell surface clustering and internalization of CT-B was clearly impaired in Cav-1 null (KO) MEFs. Arrows point at areas of cell surface clustering and internalization in wild-type (WT) MEFs. (B) MEFs after 45 min of internalization. Note that little or no cell surface clustering and internalization was apparent in Cav-1 null MEFs. In contrast, by 45 min, internalization of CT-B in wild-type MEFs was virtually complete, with a high concentration of CT-B accumulating in a perinuclear compartment (arrows).
Similar articles
- The tetraspan protein EMP2 modulates the surface expression of caveolins and glycosylphosphatidyl inositol-linked proteins.
Wadehra M, Goodglick L, Braun J. Wadehra M, et al. Mol Biol Cell. 2004 May;15(5):2073-83. doi: 10.1091/mbc.e03-07-0488. Epub 2004 Feb 20. Mol Biol Cell. 2004. PMID: 14978215 Free PMC article. - Palmitoylation of caveolin-1 at a single site (Cys-156) controls its coupling to the c-Src tyrosine kinase: targeting of dually acylated molecules (GPI-linked, transmembrane, or cytoplasmic) to caveolae effectively uncouples c-Src and caveolin-1 (TYR-14).
Lee H, Woodman SE, Engelman JA, Volonté D, Galbiati F, Kaufman HL, Lublin DM, Lisanti MP. Lee H, et al. J Biol Chem. 2001 Sep 14;276(37):35150-8. doi: 10.1074/jbc.M104530200. Epub 2001 Jul 12. J Biol Chem. 2001. PMID: 11451957 - Signal transducing molecules and glycosyl-phosphatidylinositol-linked proteins form a caveolin-rich insoluble complex in MDCK cells.
Sargiacomo M, Sudol M, Tang Z, Lisanti MP. Sargiacomo M, et al. J Cell Biol. 1993 Aug;122(4):789-807. doi: 10.1083/jcb.122.4.789. J Cell Biol. 1993. PMID: 8349730 Free PMC article. - The Caveolin genes: from cell biology to medicine.
Williams TM, Lisanti MP. Williams TM, et al. Ann Med. 2004;36(8):584-95. doi: 10.1080/07853890410018899. Ann Med. 2004. PMID: 15768830 Review. - Caveolins, caveolae, and lipid rafts in cellular transport, signaling, and disease.
Quest AF, Leyton L, Párraga M. Quest AF, et al. Biochem Cell Biol. 2004 Feb;82(1):129-44. doi: 10.1139/o03-071. Biochem Cell Biol. 2004. PMID: 15052333 Review.
Cited by
- Probing the caveolin-1 P132L mutant: critical insights into its oligomeric behavior and structure.
Rieth MD, Lee J, Glover KJ. Rieth MD, et al. Biochemistry. 2012 May 8;51(18):3911-8. doi: 10.1021/bi3001853. Epub 2012 Apr 25. Biochemistry. 2012. PMID: 22506673 Free PMC article. - Trafficking of glycosylphosphatidylinositol anchored proteins from the endoplasmic reticulum to the cell surface.
Muñiz M, Riezman H. Muñiz M, et al. J Lipid Res. 2016 Mar;57(3):352-60. doi: 10.1194/jlr.R062760. Epub 2015 Oct 8. J Lipid Res. 2016. PMID: 26450970 Free PMC article. Review. - Spatial segregation of transport and signalling functions between human endothelial caveolae and lipid raft proteomes.
Sprenger RR, Fontijn RD, van Marle J, Pannekoek H, Horrevoets AJ. Sprenger RR, et al. Biochem J. 2006 Dec 15;400(3):401-10. doi: 10.1042/BJ20060355. Biochem J. 2006. PMID: 16886909 Free PMC article. - Regulation of raft-dependent endocytosis.
Lajoie P, Nabi IR. Lajoie P, et al. J Cell Mol Med. 2007 Jul-Aug;11(4):644-53. doi: 10.1111/j.1582-4934.2007.00083.x. J Cell Mol Med. 2007. PMID: 17760830 Free PMC article. Review. - The tetraspan protein EMP2 modulates the surface expression of caveolins and glycosylphosphatidyl inositol-linked proteins.
Wadehra M, Goodglick L, Braun J. Wadehra M, et al. Mol Biol Cell. 2004 May;15(5):2073-83. doi: 10.1091/mbc.e03-07-0488. Epub 2004 Feb 20. Mol Biol Cell. 2004. PMID: 14978215 Free PMC article.
References
- Ahmed, S. N., D. A. Brown, and E. London. 1997. On the origin of sphingolipid/cholesterol-rich detergent-insoluble cell membranes: physiological concentrations of cholesterol and sphingolipid induce formation of a detergent-insoluble, liquid-ordered lipid phase in model membranes. Biochemistry 36:10944-10953. - PubMed
- Angst, B. D., C. Marcozzi, and A. Magee. 2001. The cadherin superfamily: diversity in form and function. J. Cell Sci. 114:629-641. - PubMed
- Bamezai, A., and K. L. Rock. 1991. Effect of ras-activation on the expression of GPI-anchored proteins on the plasma membrane. Oncogene 6:1445-1451. - PubMed
- Brodsky, R. A., G. L. Mukhina, K. L. Nelson, T. S. Lawrence, R. J. Jones, and J. T. Buckley. 1999. Resistance of paroxysmal nocturnal hemoglobinuria cells to the glycosylphosphatidylinositol-binding toxin aerolysin. Blood 93:1749-1756. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous