Chronic helminth infection induces alternatively activated macrophages expressing high levels of CCR5 with low interleukin-12 production and Th2-biasing ability - PubMed (original) (raw)
Chronic helminth infection induces alternatively activated macrophages expressing high levels of CCR5 with low interleukin-12 production and Th2-biasing ability
Miriam Rodríguez-Sosa et al. Infect Immun. 2002 Jul.
Abstract
Helminth infections induce Th2-type biased immune responses. Although the mechanisms involved in this phenomenon are not yet clearly defined, antigen-presenting cells (APC) could play an important role in this process. Here, we have used peritoneal macrophages (F4/80+) recruited at different times after challenge with Taenia crassiceps as APC and tested their ability to regulate Th1/Th2 differentiation. Macrophages from acute infections produced high levels of interleukin-12 (IL-12) and nitric oxide (NO), paralleled with low levels of IL-6 and prostaglandin E(2) (PGE(2)) and with the ability to induce strong antigen-specific CD4+ T-cell proliferation in response to nonrelated antigens. In contrast, macrophages from chronic infections produced higher levels of IL-6 and PGE(2) and had suppressed production of IL-12 and NO, associated with a poor ability to induce antigen-specific proliferation in CD4+ T cells. Failure to induce proliferation was not due to a deficient expression of accessory molecules, since major histocompatibility complex class II, CD40, and B7-2 were up-regulated, together with CD23 and CCR5 as infection progressed. These macrophages from chronic infections were able to bias CD4+ T cells to produce IL-4 but not gamma interferon (IFN-gamma), contrary to macrophages from acute infections. Blockade of B7-2 and IL-6 and inhibition of PGE(2) failed to restore the proliferative response in CD4+ T cells. Furthermore, studies using STAT6(-/-) mice revealed that STAT6-mediated signaling was essential for the expansion of these alternatively activated macrophages. These data demonstrate that helminth infections can induce different macrophage populations that have Th2-biasing properties.
Figures
FIG. 1.
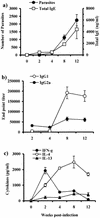
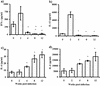
Course of T. crassiceps infection in BALB/c mice and Th1/Th2 markers. (a) Female BALB/c mice were inoculated i.p. with 20 cysticerci of T. crassiceps, and parasite loads and total IgE levels were monitored at times indicated. (b) Endpoint titers for antigen-specific antibody production of IgG1 and IgG2a. (c) Antigen-specific production of IFN-γ, IL-4, and IL-13 by splenocytes from _T. crassiceps_-infected mice as markers of Th1/Th2 environment. Data are representative of three independent experiments. Error bars represent ± standard deviations.
FIG. 2.
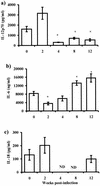
Cytokine production by macrophages in response to Th1-polarizing antigen (LPS). IL-12 (a), IL-6 (b), and IL-18 (c) production by macrophages from different weeks after T. crassiceps infection was determined by ELISA. Adherent PECs were obtained from infected mice at the times indicated, as well as from age-matched uninfected mice. Macrophages (106) were stimulated in vitro with 5 μg of LPS/ml for 48 h. Data are the mean ± standard deviation for six animals at each time point. Asterisk, P < 0.05 with respect to uninfected mice and mice at 2 weeks postinfection; ND, not determined.
FIG. 3.
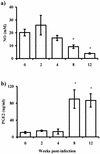
Other products of macrophages' activity are altered by T. crassiceps infection. Production of PGE2 (a) and NO (b) as measured by ELISA or Griess reaction were assayed from the same culture supernatants from Fig. 2. Asterisk, P < 0.05 with respect to uninfected mice and mice at 2 weeks postinfection.
FIG. 4.
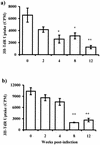
T. crassiceps infection alters the capacity of macrophages as antigen-presenting cells. Macrophages from different weeks after infection were loaded with KLH (a) or OVA (b) and cocultured with CD4+ cells (previously sensitized) for 5 days; proliferation was assayed by uptake of [3H]thymidine (3H-TdR). Data are representative of three different experiments, ± standard errors. Asterisk, P < 0.05; double asterisk, P < 0.01 (compared to uninfected mice or mice infected for 2 weeks).
FIG. 5.
T-cell-polarizing ability of alternatively activated macrophages in vivo during T. crassiceps infection. Adherent peritoneal macrophages were obtained from infected mice at the times indicated, as well as from age-matched uninfected mice. Cells (106) were loaded in vitro with 50 μg of OVA for 2 h. CD4+ T cells (2 × 106) from healthy mice previously immunized with OVA were cocultured, and 72 h later supernatants were analyzed for IFN-γ (a), IL-12 (b), IL-4 (c), and IL-6 (d). Data are representative of three independent experiments with at least four animals individually assayed. Error bars indicate ± standard deviations. Asterisk, P < 0.05 comparing with macrophages from healthy mice or early (2 weeks)-infected mice.
FIG. 6.
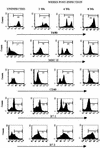
Phenotypes of peritoneal macrophages isolated from T. crassiceps infection. Cell surface expression of F4/80, MHC-II, CD40, B7-1, and B7-2 at 0, 2, 4, and 8 weeks after T. crassiceps infection are shown. Data are representative histograms of three separate experiments.
FIG. 7.
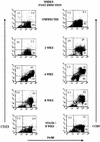
T. crassiceps infection up-regulates the expression of CD23/CCR5 in macrophages. Cell surface expression of F4/80-CD23 and F4/80-CCR5 on peritoneal macrophages obtained at different weeks after infection. Bottom, macrophages obtained from STAT6-KO mice 8 weeks after T. crassiceps infection.
FIG. 8.
Effect of fixation, blockade of IL-6/B7-2, inhibition of PGE2, and absence of STAT6 signaling of macrophages in CD4+ T-cell proliferation and cytokine production. (a) Macrophages from mice at 8 weeks postinfection were used as APC and cocultured as for Fig. 4. In addition, blocking antibodies for IL-6 and B7-2, indomethacin, fixed macrophages, and STAT6-KO macrophages were used in these cocultures. (a) Proliferation was assayed by [3H]thymidine (3H-TDR) incorporation. (b to d) Cytokine levels detected in the presence and absence of blocking antibodies, indomethacin, fixed macrophages, or STAT6-KO macrophages. Asterisk, P < 0.05 compared to isotype control. ND, not determined.
Similar articles
- Cutting edge: susceptibility to the larval stage of the helminth parasite Taenia crassiceps is mediated by Th2 response induced via STAT6 signaling.
Rodriguez-Sosa M, David JR, Bojalil R, Satoskar AR, Terrazas LI. Rodriguez-Sosa M, et al. J Immunol. 2002 Apr 1;168(7):3135-9. doi: 10.4049/jimmunol.168.7.3135. J Immunol. 2002. PMID: 11907063 - Antigen-presenting cells recruited by Brugia malayi induce Th2 differentiation of naïve CD4(+) T cells.
Loke P, MacDonald AS, Allen JE. Loke P, et al. Eur J Immunol. 2000 Apr;30(4):1127-35. doi: 10.1002/(SICI)1521-4141(200004)30:4<1127::AID-IMMU1127>3.0.CO;2-#. Eur J Immunol. 2000. PMID: 10760802 - Immune function of astrocytes.
Dong Y, Benveniste EN. Dong Y, et al. Glia. 2001 Nov;36(2):180-90. doi: 10.1002/glia.1107. Glia. 2001. PMID: 11596126 Review. - Tumor immunity: a balancing act between T cell activation, macrophage activation and tumor-induced immune suppression.
Sinha P, Clements VK, Miller S, Ostrand-Rosenberg S. Sinha P, et al. Cancer Immunol Immunother. 2005 Nov;54(11):1137-42. doi: 10.1007/s00262-005-0703-4. Epub 2005 May 5. Cancer Immunol Immunother. 2005. PMID: 15877228 Free PMC article. Review.
Cited by
- Sex-associated expression of co-stimulatory molecules CD80, CD86, and accessory molecules, PDL-1, PDL-2 and MHC-II, in F480+ macrophages during murine cysticercosis.
Togno-Peirce C, Nava-Castro K, Terrazas LI, Morales-Montor J. Togno-Peirce C, et al. Biomed Res Int. 2013;2013:570158. doi: 10.1155/2013/570158. Epub 2013 Jan 20. Biomed Res Int. 2013. PMID: 23533995 Free PMC article. - Taenia solium glutathione transferase fraction activates macrophages and favors the development of Th1-type response.
Vega-Angeles VT, Terrazas LI, Ledesma-Soto Y, Jiménez L, Landa A. Vega-Angeles VT, et al. Biosci Rep. 2019 Jan 18;39(1):BSR20181132. doi: 10.1042/BSR20181132. Print 2019 Jan 31. Biosci Rep. 2019. PMID: 30538171 Free PMC article. - Macrophage proliferation, provenance, and plasticity in macroparasite infection.
Rückerl D, Allen JE. Rückerl D, et al. Immunol Rev. 2014 Nov;262(1):113-33. doi: 10.1111/imr.12221. Immunol Rev. 2014. PMID: 25319331 Free PMC article. Review. - Tolerance and M2 (alternative) macrophage polarization are related processes orchestrated by p50 nuclear factor kappaB.
Porta C, Rimoldi M, Raes G, Brys L, Ghezzi P, Di Liberto D, Dieli F, Ghisletti S, Natoli G, De Baetselier P, Mantovani A, Sica A. Porta C, et al. Proc Natl Acad Sci U S A. 2009 Sep 1;106(35):14978-83. doi: 10.1073/pnas.0809784106. Epub 2009 Aug 17. Proc Natl Acad Sci U S A. 2009. PMID: 19706447 Free PMC article. - Immunopathology of schistosomiasis.
Wilson MS, Mentink-Kane MM, Pesce JT, Ramalingam TR, Thompson R, Wynn TA. Wilson MS, et al. Immunol Cell Biol. 2007 Feb-Mar;85(2):148-54. doi: 10.1038/sj.icb.7100014. Epub 2006 Dec 12. Immunol Cell Biol. 2007. PMID: 17160074 Free PMC article. Review.
References
- Afonso, L. C., T. M. Scharton, L. Q. Vieira, M. Wysocka, G. Trinchieri, and P. Scott. 1994. The adjuvant effect of interleukin-12 in a vaccine against Leishmania major. Science 263:235-237. - PubMed
- Aliberti, J., C. Reis e Sousa, M. Schito, S. Hieny, T. Wells, G. B. Huffnagle, and A. Sher. 2000. CCR5 provides a signal for microbial induced production of IL-12 by CD8 alpha+ dendritic cells. Nat. Immunol. 1:83-87. - PubMed
- Allen, J. E., and P. Loke. 2001. Divergent roles for macrophages in lymphatic filariasis. Parasite Immunol. 23:345-352. - PubMed
- Bliss, S. K., B. A. Butcher, and E. Y. Denkers. 2000. Rapid recruitment of neutrophils containing prestored IL-12 during microbial infection. J. Immunol. 165:4515-4521. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous